This is “Solubility and pH”, section 17.4 from the book Principles of General Chemistry (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
17.4 Solubility and pH
Learning Objective
- To understand why the solubility of many compounds depends on pH.
The solubility of many compounds depends strongly on the pH of the solution. For example, the anion in many sparingly soluble salts is the conjugate base of a weak acid that may become protonated in solution. In addition, the solubility of simple binary compounds such as oxides and sulfides, both strong bases, is often dependent on pH. In this section, we discuss the relationship between the solubility of these classes of compounds and pH.
The Effect of Acid–Base Equilibriums on the Solubility of Salts
We begin our discussion by examining the effect of pH on the solubility of a representative salt, M+A−, where A− is the conjugate base of the weak acid HA. When the salt dissolves in water, the following reaction occurs:
Equation 17.13
The anion can also react with water in a hydrolysis reaction:
Equation 17.14
Because of the reaction described in Equation 17.14, the predicted solubility of a sparingly soluble salt that has a basic anion such as S2−, PO43−, or CO32− is increased, as described in Section 17.1 "Determining the Solubility of Ionic Compounds". If instead a strong acid is added to the solution, the added H+ will react essentially completely with A− to form HA. This reaction decreases [A−], which decreases the magnitude of the ion product (Q = [M+][A−]). According to Le Châtelier’s principle, more MA will dissolve until Q = Ksp. Hence an acidic pH dramatically increases the solubility of virtually all sparingly soluble salts whose anion is the conjugate base of a weak acid. In contrast, pH has little to no effect on the solubility of salts whose anion is the conjugate base of a stronger weak acid or a strong acid, respectively (e.g., chlorides, bromides, iodides, and sulfates). For example, the hydroxide salt Mg(OH)2 is relatively insoluble in water:
Equation 17.15
When acid is added to a saturated solution that contains excess solid Mg(OH)2, the following reaction occurs, removing OH− from solution:
Equation 17.16
The overall equation for the reaction of Mg(OH)2 with acid is thus
Equation 17.17
As more acid is added to a suspension of Mg(OH)2, the equilibrium shown in Equation 17.17 is driven to the right, so more Mg(OH)2 dissolves.
Such pH-dependent solubility is not restricted to salts that contain anions derived from water. For example, CaF2 is a sparingly soluble salt:
Equation 17.18
When strong acid is added to a saturated solution of CaF2, the following reaction occurs:
Equation 17.19
Because the forward reaction decreases the fluoride ion concentration, more CaF2 dissolves to relieve the stress on the system. The net reaction of CaF2 with strong acid is thus
Equation 17.20
CaF2(s) + 2H+(aq) → Ca2+(aq) + 2HF(aq)Example 7 shows how to calculate the solubility effect of adding a strong acid to a solution of a sparingly soluble salt.
Note the Pattern
Sparingly soluble salts derived from weak acids tend to be more soluble in an acidic solution.
Example 7
Lead oxalate (PbC2O4), lead iodide (PbI2), and lead sulfate (PbSO4) are all rather insoluble, with Ksp values of 4.8 × 10−10, 9.8 × 10−9, and 2.53 × 10−8, respectively. What effect does adding a strong acid, such as perchloric acid, have on their relative solubilities?
Given: Ksp values for three compounds
Asked for: relative solubilities in acid solution
Strategy:
Write the balanced chemical equation for the dissolution of each salt. Because the strongest conjugate base will be most affected by the addition of strong acid, determine the relative solubilities from the relative basicity of the anions.
Solution:
The solubility equilibriums for the three salts are as follows:
The addition of a strong acid will have the greatest effect on the solubility of a salt that contains the conjugate base of a weak acid as the anion. Because HI is a strong acid, we predict that adding a strong acid to a saturated solution of PbI2 will not greatly affect its solubility; the acid will simply dissociate to form H+(aq) and the corresponding anion. In contrast, oxalate is the fully deprotonated form of oxalic acid (HO2CCO2H), which is a weak diprotic acid (pKa1 = 1.23 and pKa2 = 4.19). Consequently, the oxalate ion has a significant affinity for one proton and a lower affinity for a second proton. Adding a strong acid to a saturated solution of lead oxalate will result in the following reactions:
These reactions will decrease [C2O42−], causing more lead oxalate to dissolve to relieve the stress on the system.The pKa of HSO4− (1.99) is similar in magnitude to the pKa1 of oxalic acid, so adding a strong acid to a saturated solution of PbSO4 will result in the following reaction:
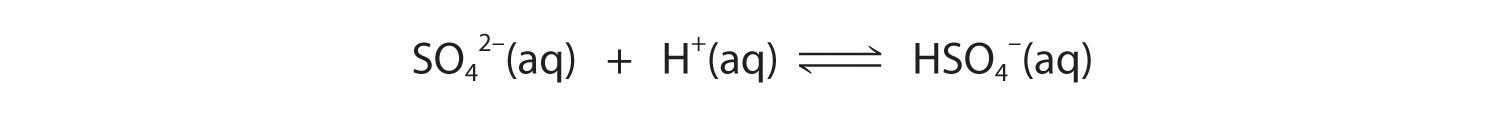
Because HSO4− has a pKa of 1.99, this reaction will lie largely to the left as written. Consequently, we predict that the effect of added strong acid on the solubility of PbSO4 will be significantly less than for PbC2O4.
Exercise
Which of the following insoluble salts—AgCl, Ag2CO3, Ag3PO4, and/or AgBr—will be substantially more soluble in 1.0 M HNO3 than in pure water?
Answer: Ag2CO3 and Ag3PO4
Caves and their associated pinnacles and spires of stone provide one of the most impressive examples of pH-dependent solubility equilibriums (part (a) in Figure 17.6 "The Chemistry of Cave Formation"). Perhaps the most familiar caves are formed from limestone, such as Carlsbad Caverns in New Mexico, Mammoth Cave in Kentucky, and Luray Caverns in Virginia. The primary reactions that are responsible for the formation of limestone caves are as follows:
Equation 17.21
Equation 17.22
Equation 17.23
Figure 17.6 The Chemistry of Cave Formation

(a) This cave in Campanet, Mallorca, Spain, and its associated formations are examples of pH-dependent solubility equilibriums. (b) A cave forms when groundwater containing atmospheric CO2, forming an acidic solution, dissolves limestone (CaCO3) in a process that may take tens of thousands of years. As groundwater seeps into a cave, water evaporates from the solution of CaCO3 in CO2-rich water, producing a supersaturated solution and a shift in equilibrium that causes precipitation of the CaCO3. The deposited limestone eventually forms stalactites and stalagmites.
Limestone deposits that form caves consist primarily of CaCO3 from the remains of living creatures such as clams and corals, which used it for making structures such as shells. When a saturated solution of CaCO3 in CO2-rich water rises toward Earth’s surface or is otherwise heated, CO2 gas is released as the water warms. CaCO3 then precipitates from the solution according to the following equation (part (b) in Figure 17.6 "The Chemistry of Cave Formation"):
Equation 17.24
The forward direction is the same reaction that produces the solid called scale in teapots, coffee makers, water heaters, boilers, and other places where hard water is repeatedly heated.
When groundwater-containing atmospheric CO2 (Equation 17.21 and Equation 17.22) finds its way into microscopic cracks in the limestone deposits, CaCO3 dissolves in the acidic solution in the reverse direction of Equation 17.24. The cracks gradually enlarge from 10–50 µm to 5–10 mm, a process that can take as long as 10,000 yr. Eventually, after about another 10,000 yr, a cave forms. Groundwater from the surface seeps into the cave and clings to the ceiling, where the water evaporates and causes the equilibrium in Equation 17.24 to shift to the right. A circular layer of solid CaCO3 is deposited, which eventually produces a long, hollow spire of limestone called a stalactite that grows down from the ceiling. Below, where the droplets land when they fall from the ceiling, a similar process causes another spire, called a stalagmite, to grow up. The same processes that carve out hollows below ground are also at work above ground, in some cases producing fantastically convoluted landscapes like that of Yunnan Province in China (Figure 17.7 "Solubility Equilibriums in the Formation of Karst Landscapes").
Figure 17.7 Solubility Equilibriums in the Formation of Karst Landscapes

Landscapes such as the steep limestone pinnacles of the Stone Forest in Yunnan Province, China, are formed from the same process that produces caves and their associated formations.
Acidic, Basic, and Amphoteric Oxides and Hydroxides
One of the earliest classifications of substances was based on their solubility in acidic versus basic solution, which led to the classification of oxides and hydroxides as being either basic or acidic. Basic oxidesAn oxide that reacts with water to produce a basic solution or dissolves readily in aqueous acid. and hydroxides either react with water to produce a basic solution or dissolve readily in aqueous acid. Acidic oxidesAn oxide that reacts with water to produce an acidic solution or dissolves in aqueous base. or hydroxides either react with water to produce an acidic solution or are soluble in aqueous base. As shown in Figure 17.8 "Classification of the Oxides of the Main Group Elements According to Their Acidic or Basic Character", there is a clear correlation between the acidic or the basic character of an oxide and the position of the element combined with oxygen in the periodic table. Oxides of metallic elements are generally basic oxides, and oxides of nonmetallic elements are acidic oxides. Compare, for example, the reactions of a typical metal oxide, cesium oxide, and a typical nonmetal oxide, sulfur trioxide, with water:
Equation 17.25
Equation 17.26
Cesium oxide reacts with water to produce a basic solution of cesium hydroxide, whereas sulfur trioxide reacts with water to produce a solution of sulfuric acid—very different behaviors indeed!
Note the Pattern
Metal oxides generally react with water to produce basic solutions, whereas nonmetal oxides produce acidic solutions.
The difference in reactivity is due to the difference in bonding in the two kinds of oxides. Because of the low electronegativity of the metals at the far left in the periodic table, their oxides are best viewed as containing discrete Mn+ cations and O2− anions. At the other end of the spectrum are nonmetal oxides; due to their higher electronegativities, nonmetals form oxides with covalent bonds to oxygen. Because of the high electronegativity of oxygen, however, the covalent bond between oxygen and the other atom, E, is usually polarized: Eδ+–Oδ−. The atom E in these oxides acts as a Lewis acid that reacts with the oxygen atom of water to produce an oxoacid. Oxides of metals in high oxidation states also tend to be acidic oxides for the same reason: they contain covalent bonds to oxygen. An example of an acidic metal oxide is MoO3, which is insoluble in both water and acid but dissolves in strong base to give solutions of the molybdate ion (MoO42−):
Equation 17.27
MoO3(s) + 2OH−(aq) → MoO42−(aq) + H2O(l)As shown in Figure 17.8 "Classification of the Oxides of the Main Group Elements According to Their Acidic or Basic Character", there is a gradual transition from basic metal oxides to acidic nonmetal oxides as we go from the lower left to the upper right in the periodic table, with a broad diagonal band of oxides of intermediate character separating the two extremes. Many of the oxides of the elements in this diagonal region of the periodic table are soluble in both acidic and basic solutions; consequently, they are called amphoteric oxidesAn oxide that can dissolve in acid to produce water and dissolve in base to produce a soluble complex. (from the Greek ampho, meaning “both,” as in amphiprotic, which was defined in Chapter 16 "Aqueous Acid–Base Equilibriums", Section 16.1 "The Autoionization of Water"). Amphoteric oxides either dissolve in acid to produce water or dissolve in base to produce a soluble complex. As shown in Figure 17.9 "Chromium(III) Hydroxide [Cr(OH)", for example, mixing the amphoteric oxide Cr(OH)3 (also written as Cr2O3·3H2O) with water gives a muddy, purple-brown suspension. Adding acid causes the Cr(OH)3 to dissolve to give a bright violet solution of Cr3+(aq), which contains the [Cr(H2O)6]3+ ion, whereas adding strong base gives a green solution of the [Cr(OH)4]− ion. The chemical equations for the reactions are as follows:
Equation 17.28
Equation 17.29
Figure 17.8 Classification of the Oxides of the Main Group Elements According to Their Acidic or Basic Character
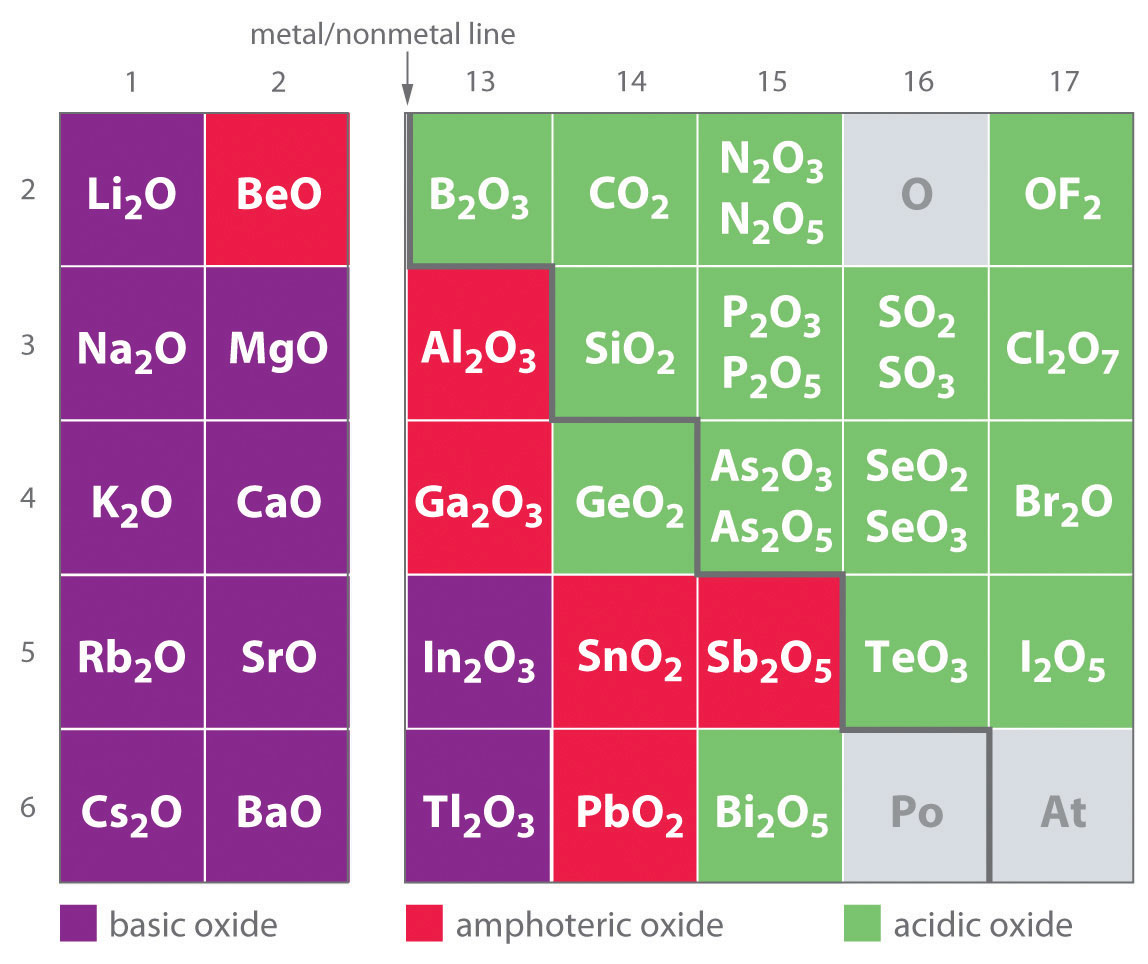
There is a gradual transition from basic oxides to acidic oxides from the lower left to the upper right in the periodic table. Oxides of metallic elements are generally basic oxides, which either react with water to form a basic solution or dissolve in aqueous acid. In contrast, oxides of nonmetallic elements are acidic oxides, which either react with water to form an acidic solution or are soluble in aqueous base. Oxides of intermediate character, called amphoteric oxides, are located along a diagonal line between the two extremes. Amphoteric oxides either dissolve in acid to produce water or dissolve in base to produce a soluble complex ion. (Radioactive elements are not classified.)
Figure 17.9 Chromium(III) Hydroxide [Cr(OH)3 or Cr2O3·3H2O] Is an Example of an Amphoteric Oxide
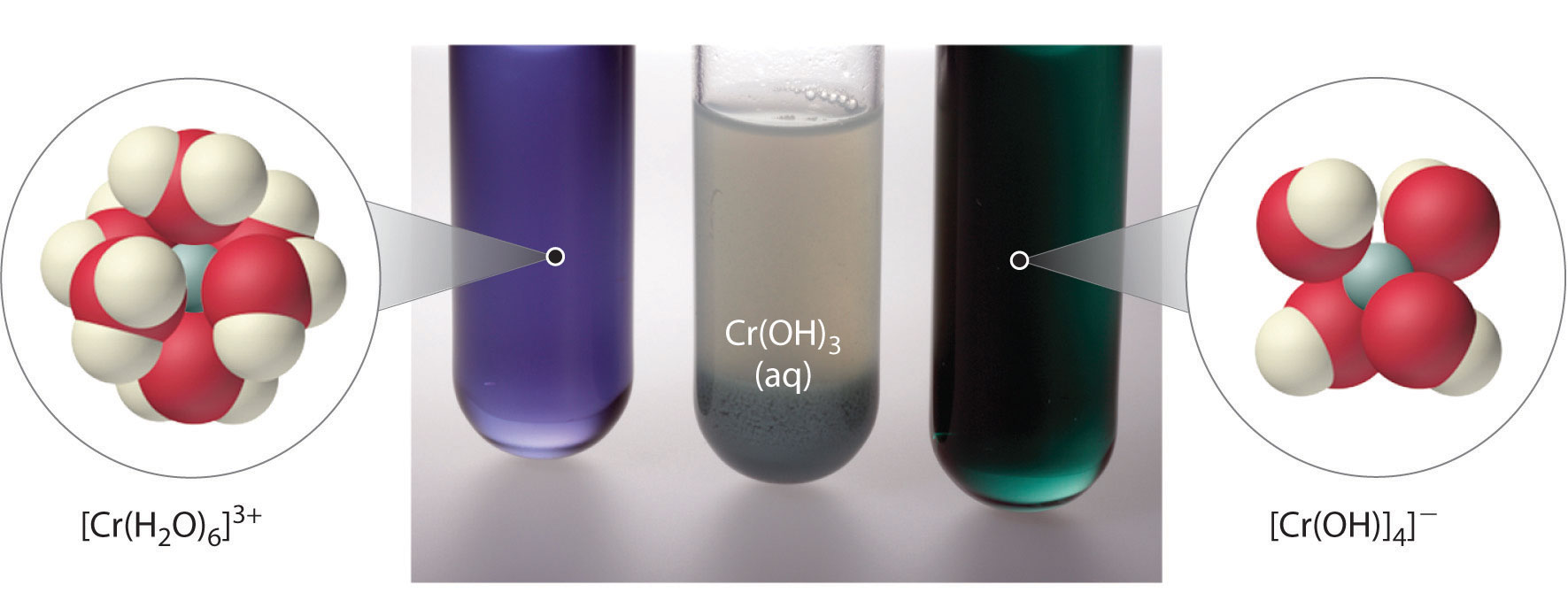
All three beakers originally contained a suspension of brownish purple Cr(OH)3(s) (center). When concentrated acid (6 M H2SO4) was added to the beaker on the left, Cr(OH)3 dissolved to produce violet [Cr(H2O)6]3+ ions and water. The addition of concentrated base (6 M NaOH) to the beaker on the right caused Cr(OH)3 to dissolve, producing green [Cr(OH)4]−ions.
Example 8
Aluminum hydroxide, written as either Al(OH)3 or Al2O3·3H2O, is amphoteric. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base.
Given: amphoteric compound
Asked for: dissolution reactions in acid and base
Strategy:
Using Equation 17.28 and Equation 17.29 as a guide, write the dissolution reactions in acid and base solutions.
Solution:
-
An acid donates protons to hydroxide to give water and the hydrated metal ion, so aluminum hydroxide, which contains three OH− ions per Al, needs three H+ ions:
Al(OH)3(s) + 3H+(aq) → Al3+(aq) + 3H2O(l)In aqueous solution, Al3+ forms the complex ion [Al(H2O)6]3+.
-
In basic solution, OH− is added to the compound to produce a soluble and stable poly(hydroxo) complex:
Al(OH)3(s) + OH−(aq) → [Al(OH)4]−(aq)
Exercise
Copper(II) hydroxide, written as either Cu(OH)2 or CuO·H2O, is amphoteric. Write chemical equations that describe the dissolution of cupric hydroxide both in an acid and in a base.
Answer:
Cu(OH)2(s) + 2H+(aq) → Cu2+(aq) + 2H2O(l) Cu(OH)2(s) + 2OH−(aq) → [Cu(OH)4]2−(aq)Selective Precipitation Using pH
Many dissolved metal ions can be separated by the selective precipitation of the cations from solution under specific conditions. In this technique, pH is often used to control the concentration of the anion in solution, which controls which cations precipitate.
Note the Pattern
The concentration of anions in solution can often be controlled by adjusting the pH, thereby allowing the selective precipitation of cations.
Suppose, for example, we have a solution that contains 1.0 mM Zn2+ and 1.0 mM Cd2+ and want to separate the two metals by selective precipitation as the insoluble sulfide salts, ZnS and CdS. The relevant solubility equilibriums can be written as follows:
Equation 17.30
Equation 17.31
Because the S2− ion is quite basic and reacts extensively with water to give HS− and OH−, the solubility equilibriums are more accurately written as rather than Here we use the simpler form involving S2−, which is justified because we take the reaction of S2− with water into account later in the solution, arriving at the same answer using either equilibrium equation.
The sulfide concentrations needed to cause ZnS and CdS to precipitate are as follows:
Equation 17.32
Equation 17.33
Thus sulfide concentrations between 1.6 × 10−21 M and 8.0 × 10−24 M will precipitate CdS from solution but not ZnS. How do we obtain such low concentrations of sulfide? A saturated aqueous solution of H2S contains 0.10 M H2S at 20°C. The pKa1 for H2S is 6.97, and pKa2 corresponding to the formation of [S2−] is 12.90. The equations for these reactions are as follows:
Equation 17.34
We can show that the concentration of S2− is 1.3 × 10−13 by comparing Ka1 and Ka2 and recognizing that the contribution to [H+] from the dissociation of HS− is negligible compared with [H+] from the dissociation of H2S. Thus substituting 0.10 M in the equation for Ka1 for the concentration of H2S, which is essentially constant regardless of the pH, gives the following:
Equation 17.35
Substituting this value for [H+] and [HS−] into the equation for Ka2,
Although [S2−] in an H2S solution is very low (1.3 × 10−13 M), bubbling H2S through the solution until it is saturated would precipitate both metal ions because the concentration of S2− would then be much greater than 1.6 × 10−21 M. Thus we must adjust [S2−] to stay within the desired range. The most direct way to do this is to adjust [H+] by adding acid to the H2S solution (recall Le Châtelier's principle), thereby driving the equilibrium in Equation 17.34 to the left. The overall equation for the dissociation of H2S is as follows:
Equation 17.36
Now we can use the equilibrium constant K for the overall reaction, which is the product of Ka1 and Ka2, and the concentration of H2S in a saturated solution to calculate the H+ concentration needed to produce [S2−] of 1.6 × 10−21 M:
Equation 17.37
Equation 17.38
Thus adding a strong acid such as HCl to make the solution 0.94 M in H+ will prevent the more soluble ZnS from precipitating while ensuring that the less soluble CdS will precipitate when the solution is saturated with H2S.
Example 9
A solution contains 0.010 M Ca2+ and 0.010 M La3+. What concentration of HCl is needed to precipitate La2(C2O4)3·9H2O but not Ca(C2O4)·H2O if the concentration of oxalic acid is 1.0 M? Ksp values are 2.32 × 10−9 for Ca(C2O4) and 2.5 × 10−27 for La2(C2O4)3; pKa1 = 1.25 and pKa2 = 3.81 for oxalic acid.
Given: concentrations of cations, Ksp values, and concentration and pKa values for oxalic acid
Asked for: concentration of HCl needed for selective precipitation of La2(C2O4)3
Strategy:
A Write each solubility product expression and calculate the oxalate concentration needed for precipitation to occur. Determine the concentration range needed for selective precipitation of La2(C2O4)3·9H2O.
B Add the equations for the first and second dissociations of oxalic acid to get an overall equation for the dissociation of oxalic acid to oxalate. Substitute the [ox2−] needed to precipitate La2(C2O4)3·9H2O into the overall equation for the dissociation of oxalic acid to calculate the required [H+].
Solution:
A Because the salts have different stoichiometries, we cannot directly compare the magnitudes of the solubility products. Instead, we must use the equilibrium constant expression for each solubility product to calculate the concentration of oxalate needed for precipitation to occur. Using ox2− for oxalate, we write the solubility product expression for calcium oxalate as follows:
The expression for lanthanum oxalate is as follows:
Thus lanthanum oxalate is less soluble and will selectively precipitate when the oxalate concentration is between 2.9 × 10−8 M and 2.32 × 10−7 M.
B To prevent Ca2+ from precipitating as calcium oxalate, we must add enough H+ to give a maximum oxalate concentration of 2.32 × 10−7 M. We can calculate the required [H+] by using the overall equation for the dissociation of oxalic acid to oxalate:
Substituting the desired oxalate concentration into the equilibrium constant expression,
Thus adding enough HCl to give [H+] = 6.1 M will cause only La2(C2O4)3·9H2O to precipitate from the solution.
Exercise
A solution contains 0.015 M Fe2+ and 0.015 M Pb2+. What concentration of acid is needed to ensure that Pb2+ precipitates as PbS in a saturated solution of H2S, but Fe2+ does not precipitate as FeS? Ksp values are 6.3 × 10−18 for FeS and 8.0 × 10−28 for PbS.
Answer: 0.018 M H+
Summary
The anion in many sparingly soluble salts is the conjugate base of a weak acid. At low pH, protonation of the anion can dramatically increase the solubility of the salt. Oxides can be classified as acidic oxides or basic oxides. Acidic oxides either react with water to give an acidic solution or dissolve in strong base; most acidic oxides are nonmetal oxides or oxides of metals in high oxidation states. Basic oxides either react with water to give a basic solution or dissolve in strong acid; most basic oxides are oxides of metallic elements. Oxides or hydroxides that are soluble in both acidic and basic solutions are called amphoteric oxides. Most elements whose oxides exhibit amphoteric behavior are located along the diagonal line separating metals and nonmetals in the periodic table. In solutions that contain mixtures of dissolved metal ions, the pH can be used to control the anion concentration needed to selectively precipitate the desired cation.
Key Takeaway
- The anion in sparingly soluble salts is often the conjugate base of a weak acid that may become protonated in solution, so the solubility of simple oxides and sulfides, both strong bases, often depends on pH.
Conceptual Problems
-
Which of the following will show the greatest increase in solubility if 1 M HNO3 is used instead of distilled water? Explain your reasoning.
- CuCl2
- K[Pb(OH)3]
- Ba(CH3CO2)2
- CaCO3
-
Of the compounds Sn(CH3CO2)2 and SnS, one is soluble in dilute HCl and the other is soluble only in hot, concentrated HCl. Which is which? Provide a reasonable explanation.
-
Where in the periodic table do you expect to find elements that form basic oxides? Where do you expect to find elements that form acidic oxides?
-
Because water can autoionize, it reacts with oxides either as a base (as OH−) or as an acid (as H3O+). Do you expect oxides of elements in high oxidation states to be more acidic (reacting with OH−) or more basic (reacting with H3O+) than the corresponding oxides in low oxidation states? Why?
-
Given solid samples of CrO, Cr2O3, and CrO3, which would you expect to be the most acidic (reacts most readily with OH−)? Which would be the most basic (reacts most readily with H3O+)? Why?
-
Which of these elements—Be, B, Al, N, Se, In, Tl, Pb—do you expect to form an amphoteric oxide? Why?
Numerical Problems
-
A 1.0 L solution contains 1.98 M Al(NO3)3. What are [OH−] and [H+]? What pH is required to precipitate the cation as Al(OH)3? Ksp = 1.3 × 10−33 and Ka = 1.05 × 10−5 for the hydrated Al3+ ion.
-
A 1.0 L solution contains 2.03 M CoCl2. What is [H+]? What pH is required to precipitate the cation as Co(OH)2? Ksp = 5.92 × 10−15 and Ka = 1.26 × 10−9 for the hydrated Co2+ ion.
-
Given 100 mL of a solution that contains 0.80 mM Ag+ and 0.80 mM Cu+, can the two metals be separated by selective precipitation as the insoluble bromide salts by adding 10 mL of an 8.0 mM solution of KBr? Ksp values are 6.27 × 10−9 for CuBr and 5.35 × 10−13 for AgBr. What maximum [Br−] will separate the ions?
-
Given 100 mL of a solution that is 1.5 mM in Tl+, Zn2+, and Ni2+, which ions can be separated from solution by adding 5.0 mL of a 12.0 mM solution of Na2C2O4?
Precipitate K sp Tl2C2O4 2 × 10−4 ZnC2O4·2H2O 1.38 × 10−9 NiC2O4 4 × 10−10 How many milliliters of 12.0 mM Na2C2O4 should be added to separate Tl+ and Zn2+ from Ni2+?
Answers
-
[H+] = 4.56 × 10−3; [OH−] = 2.19 × 10−12; pH = 2.94
-
-
No; both metal ions will precipitate; AgBr will precipitate as Br− is added, and CuBr will begin to precipitate at [Br−] = 8.6 × 10−6 M.
-




