This is “Alkynes”, section 13.6 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
13.6 Alkynes
Learning Objectives
- Describe the general physical and chemical properties of alkynes.
- Name alkynes given formulas and write formulas for alkynes given names.
The simplest alkyne—a hydrocarbon with carbon-to-carbon triple bond—has the molecular formula C2H2 and is known by its common name—acetylene (Figure 13.5 "Ball-and-Spring Model of Acetylene"). Its structure is H–C≡C–H.
Figure 13.5 Ball-and-Spring Model of Acetylene
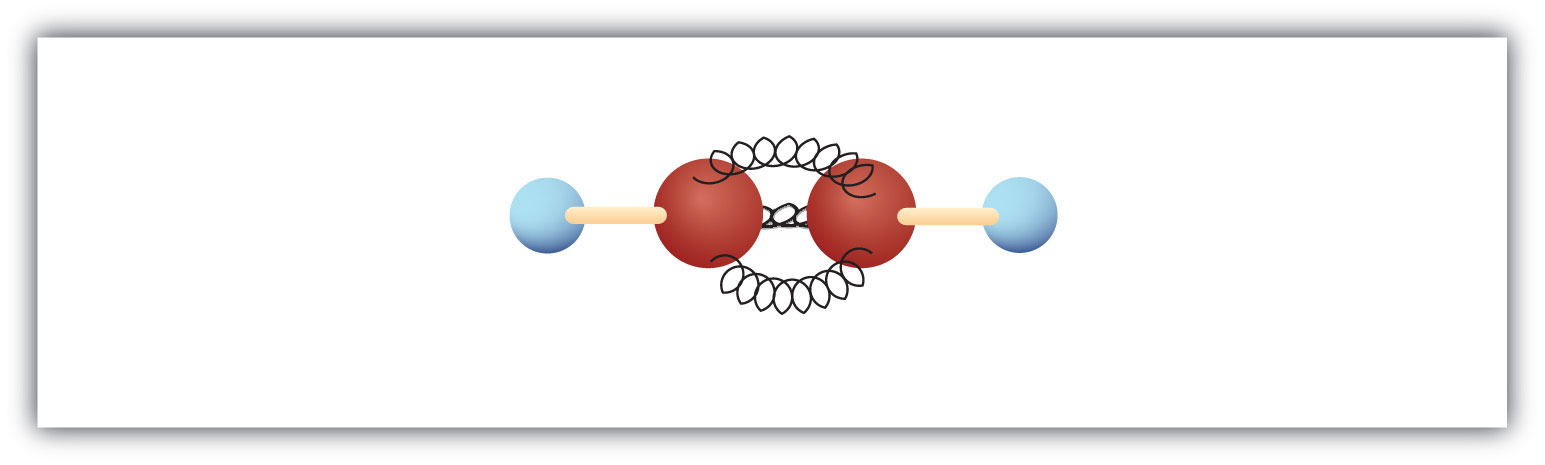
Acetylene (ethyne) is the simplest member of the alkyne family.
Note
Acetylene is used in oxyacetylene torches for cutting and welding metals. The flame from such a torch can be very hot. Most acetylene, however, is converted to chemical intermediates that are used to make vinyl and acrylic plastics, fibers, resins, and a variety of other products.
Alkynes are similar to alkenes in both physical and chemical properties. For example, alkynes undergo many of the typical addition reactions of alkenes. The International Union of Pure and Applied Chemistry (IUPAC) names for alkynes parallel those of alkenes, except that the family ending is -yne rather than -ene. The IUPAC name for acetylene is ethyne. The names of other alkynes are illustrated in the following exercises.
Concept Review Exercises
-
Briefly identify the important differences between an alkene and an alkyne. How are they similar?
-
The alkene (CH3)2CHCH2CH=CH2 is named 4-methyl-1-pentene. What is the name of (CH3)2CHCH2C≡CH?
-
Do alkynes show cis-trans isomerism? Explain.
Answers
-
Alkenes have double bonds; alkynes have triple bonds. Both undergo addition reactions.
-
4-methyl-1-pentyne
-
No; a triply bonded carbon atom can form only one other bond. It would have to have two groups attached to show cis-trans isomerism.
Key Takeaway
- Alkynes are hydrocarbons with carbon-to-carbon triple bonds and properties much like those of alkenes.
Exercises
-
Draw the structure for each compound.
- acetylene
- 3-methyl-1-hexyne
-
Draw the structure for each compound.
- 4-methyl-2-hexyne
- 3-octyne
-
Name each alkyne.
- CH3CH2CH2C≡CH
- CH3CH2CH2C≡CCH3
Answers
-
- H–C≡C–H
-
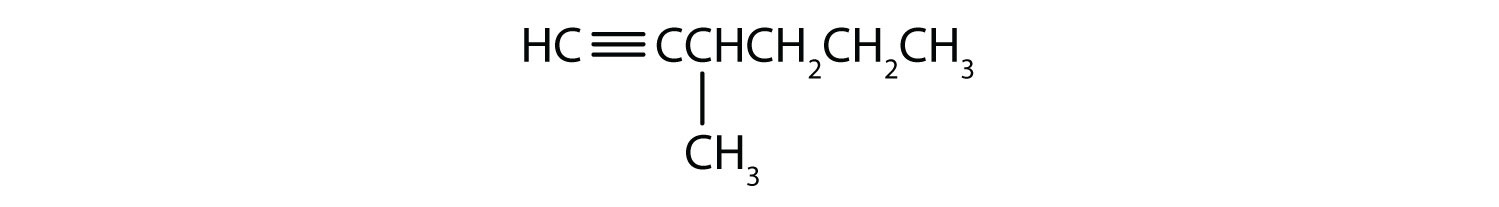
-
-
- 1-pentyne
- 2-hexyne




