This is “Polymers”, section 13.5 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
13.5 Polymers
Learning Objective
- Draw structures for monomers that can undergo addition polymerization and for four-monomer-unit sections of an addition polymer.
The most important commercial reactions of alkenes are polymerizations, reactions in which small molecules, referred to in general as monomersA small molecule that can be combined with other small molecules to make polymers. (from the Greek monos, meaning “one,” and meros, meaning “parts”), are assembled into giant molecules referred to as polymersA giant molecule formed by the combination of monomers in a repeating manner. (from the Greek poly, meaning “many,” and meros, meaning “parts”). A polymer is as different from its monomer as a long strand of spaghetti is from a tiny speck of flour. For example, polyethylene, the familiar waxy material used to make plastic bags, is made from the monomer ethylene—a gas.
The Production of Polyethylene
(click to see video)Polyethylene pellets are melted, formed into a giant bubble, and then made into a film that is used in packaging, consumer products, and food services.
There are two general types of polymerization reactions: addition polymerization and condensation polymerization. (For more information about condensation polymerization, see Chapter 15 "Organic Acids and Bases and Some of Their Derivatives", Section 15.8 "Preparation of Esters".) In addition polymerizationA reaction in which monomers add to one another to produce a polymeric product that contains all the atoms of the starting monomers., the monomers add to one another in such a way that the polymer contains all the atoms of the starting monomers. Ethylene molecules are joined together in long chains. The polymerization can be represented by the reaction of a few monomer units:
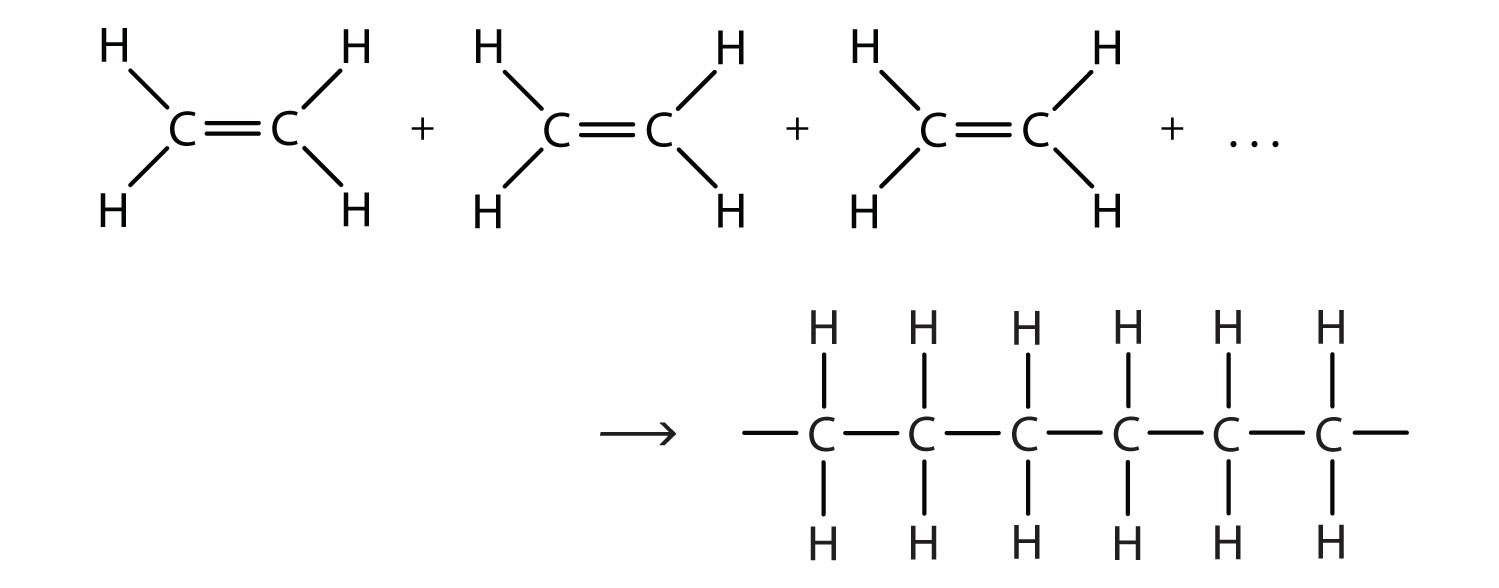
The bond lines extending at the ends in the formula of the product indicate that the structure extends for many units in each direction. Notice that all the atoms—two carbon atoms and four hydrogen atoms—of each monomer molecule are incorporated into the polymer structure. Because displays such as the one above are cumbersome, the polymerization is often abbreviated as follows:
Note
Many natural materials—such as proteins, cellulose and starch, and complex silicate minerals—are polymers. (For more information about proteins and cellulose/starch, see Chapter 18 "Amino Acids, Proteins, and Enzymes", Section 18.4 "Proteins", and Chapter 16 "Carbohydrates", Section 16.7 "Polysaccharides", respectively.) Artificial fibers, films, plastics, semisolid resins, and rubbers are also polymers. More than half the compounds produced by the chemical industry are synthetic polymers.
Some common addition polymers are listed in Table 13.2 "Some Addition Polymers". Note that all the monomers have carbon-to-carbon double bonds. Many polymers are mundane (e.g., plastic bags, food wrap, toys, and tableware), but there are also polymers that conduct electricity, have amazing adhesive properties, or are stronger than steel but much lighter in weight.
Table 13.2 Some Addition Polymers
| Monomer | Polymer | Polymer Name | Some Uses |
|---|---|---|---|
| CH2=CH2 | ~CH2CH2CH2CH2CH2CH2~ | polyethylene | plastic bags, bottles, toys, electrical insulation |
| CH2=CHCH3 |
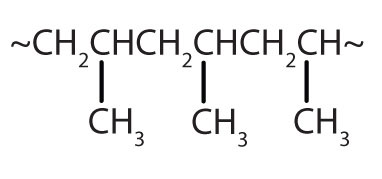
|
polypropylene | carpeting, bottles, luggage, exercise clothing |
| CH2=CHCl |
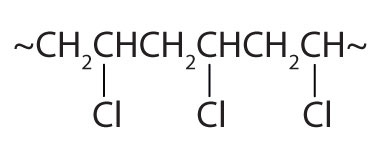
|
polyvinyl chloride | bags for intravenous solutions, pipes, tubing, floor coverings |
| CF2=CF2 | ~CF2CF2CF2CF2CF2CF2~ | polytetrafluoroethylene | nonstick coatings, electrical insulation |
Medical Uses of Polymers
An interesting use of polymers is the replacement of diseased, worn out, or missing parts in the body. For example, about a 250,000 hip joints and 500,000 knees are replaced in US hospitals each year. The artificial ball-and-socket hip joints are made of a special steel (the ball) and plastic (the socket). People crippled by arthritis or injuries gain freedom of movement and relief from pain. Patients with heart and circulatory problems can be helped by replacing worn out heart valves with parts based on synthetic polymers. These are only a few of the many biomedical uses of polymers.
Figure 13.4 Hip Joint Replacement
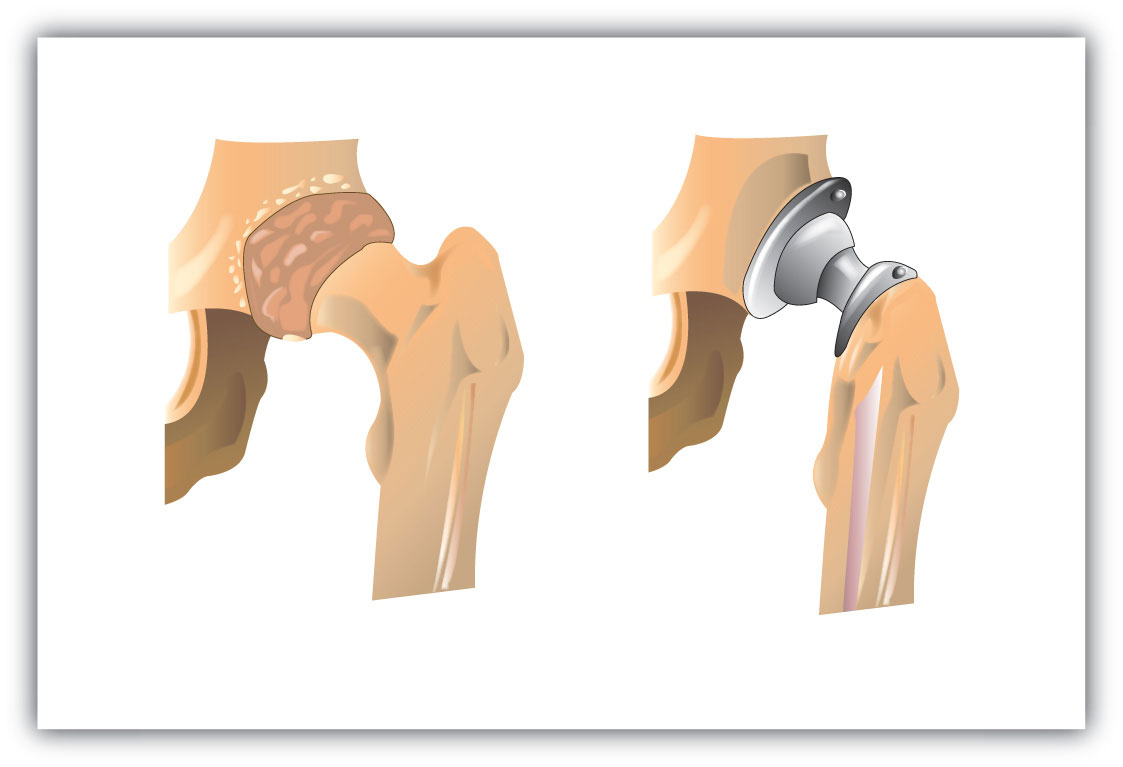
Synthetic polymers are an important part of a hip joint replacement. The hip is much like a ball-and-socket joint, and total hip replacements mimic this with a metal ball that fits in a plastic cup.
Concept Review Exercises
-
What is a monomer? What is a polymer? How do polymer molecules differ from the molecules we have discussed in earlier sections of this chapter?
-
What is addition polymerization? What structural feature usually characterizes molecules used as monomers in addition polymerization?
-
What is the molecular formula of a polymer molecule formed by the addition polymerization of 175 molecules of vinyl chloride (CH2=CHCl)?
Answers
-
Monomers are small molecules that can be assembled into giant molecules referred to as polymers, which are much larger than the molecules we discussed earlier in this chapter.
-
In addition polymerization, the monomers add to one another in such a way that the polymer contains all the atoms of the starting monomers.
-
C350H525Cl175
Key Takeaway
- Molecules having carbon-to-carbon double bonds can undergo addition polymerization.
Exercises
-
Write the condensed structural formula of the monomer from which Saran is formed. A segment of the Saran molecule has the following structure: CH2CCl2CH2CCl2CH2CCl2CH2CCl2.
-
Write the condensed structural formula for the section of a molecule formed from four units of the monomer CH2=CHF.
Answer
-
H2C=CCl2
-




