This is “Periodic Trends and the s-Block Elements”, chapter 21 from the book Principles of General Chemistry (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
Chapter 21 Periodic Trends and the s-Block Elements
In previous chapters, we used the principles of chemical bonding, thermodynamics, and kinetics to provide a conceptual framework for understanding the chemistry of the elements. Beginning in Chapter 21 "Periodic Trends and the ", we use the periodic table to guide our discussion of the properties and reactions of the elements and the synthesis and uses of some of their commercially important compounds. We begin this chapter with a review of periodic trends as an introduction, and then we describe the chemistry of hydrogen and the other s-block elements. In Chapter 22 "The ", we consider the chemistry of the p-block elements; Chapter 23 "The " presents the transition metals, in which the d-subshell is being filled. In this chapter, you will learn why potassium chloride is used as a substitute for sodium chloride in a low-sodium diet, why cesium is used as a photosensor, why the heating elements in electric ranges are coated with magnesium oxide, and why exposure to a radioactive isotope of strontium is more dangerous for children than for adults.
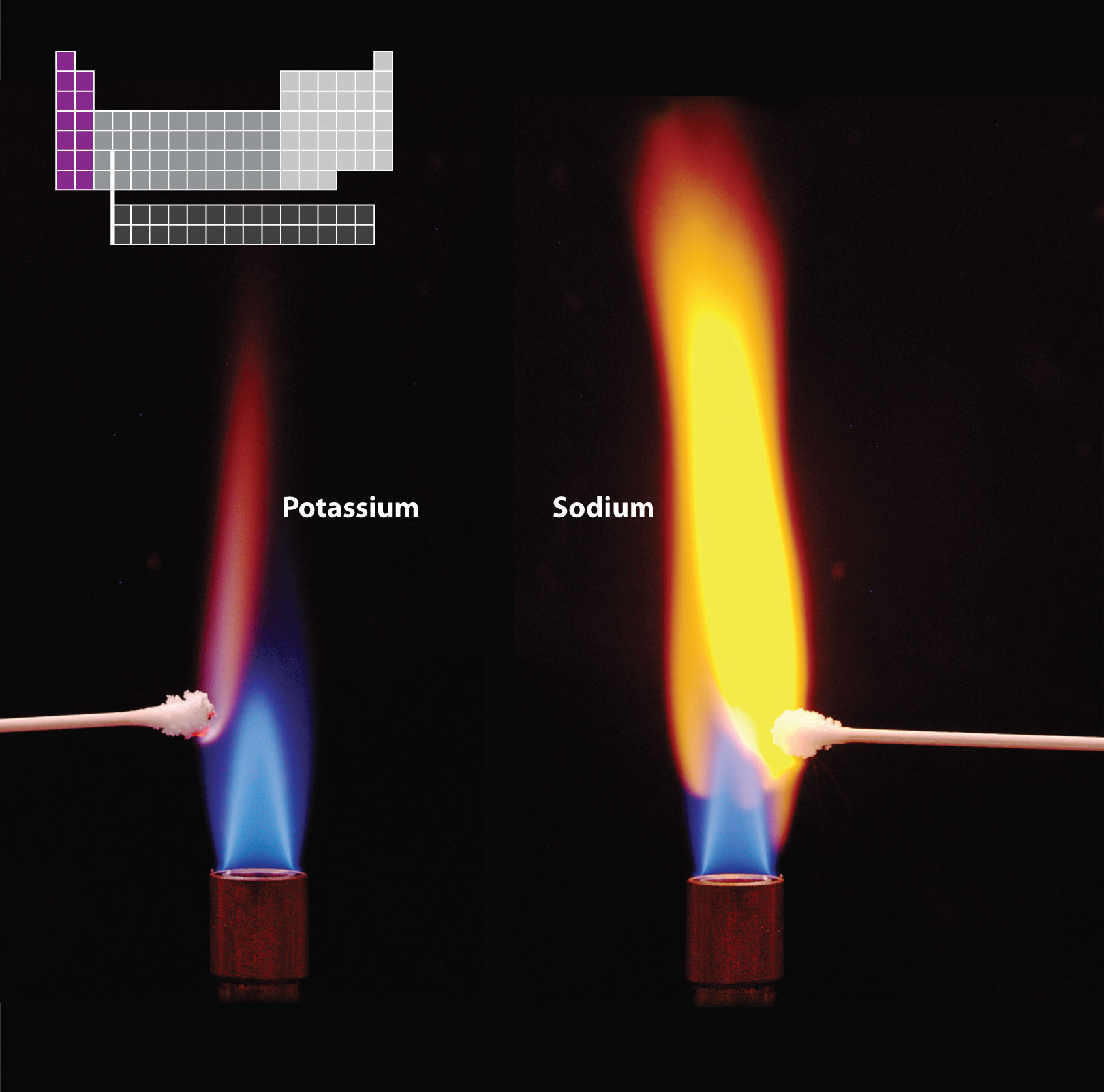
Flame tests. Heating a compound in a very hot flame results in the formation of its component atoms in electronically excited states. When an excited atom decays to the ground state, it emits light (Chapter 6 "The Structure of Atoms"). Each element emits light at characteristic frequencies. Flame tests are used to identify many elements based on the color of light emitted in the visible region of the electromagnetic spectrum. As shown here, sodium compounds produce an intense yellow light, whereas potassium compounds produce a crimson color.




