This is “End-of-Chapter Material”, section 9.5 from the book Principles of General Chemistry (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
9.5 End-of-Chapter Material
Application Problems
-
♦ Sulfur hexafluoride (SF6) is a very stable gas that is used in a wide range of applications because it is nontoxic, nonflammable, and noncorrosive. Unfortunately, it is also a very powerful “greenhouse gas” that is about 22,000 times more effective at causing global warming than the same mass of CO2.
- Draw the Lewis electron structure of SF6 and determine the number of electron groups around the central atom, the molecular geometry, and the hybridization of the central atom.
- Suggest a reason for the extremely high stability of SF6.
- Despite its rather high molecular mass (146.06 g/mol) and highly polar S–F bonds, SF6 is a gas at room temperature (boiling point = −63°C). Why?
-
♦ The elevated concentrations of chlorine monoxide (ClO) that accompany ozone depletions in Earth’s atmosphere can be explained by a sequence of reactions. In the first step, chlorine gas is split into chlorine atoms by sunlight. Each chlorine atom then catalyzes the decomposition of ozone through a chlorine monoxide intermediate.
- Write balanced chemical equations showing this sequence of reactions.
- Sketch the molecular orbital energy-level diagram of ClO.
- Does ClO contain any unpaired electrons?
- Based on your molecular orbital diagram, is ClO likely to be a stable species? Explain your answer.
-
♦ Saccharin is an artificial sweetener that was discovered in 1879. For several decades, it was used by people who had to limit their intake of sugar for medical reasons. Because it was implicated as a carcinogen in 1977, however, warning labels are now required on foods and beverages containing saccharin. The structure of this sweetener is as follows:
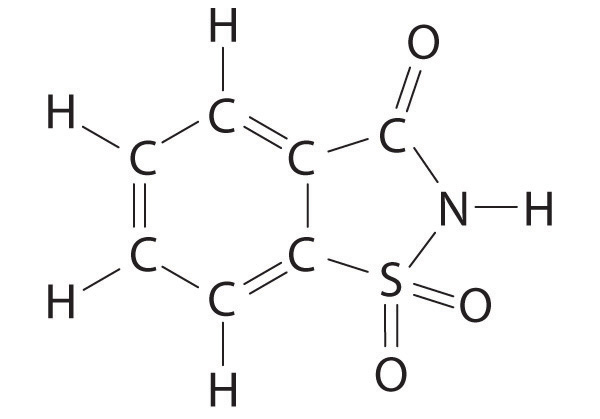
- Give the hybridization of all five atoms shown in bold in the structure. Note: all five atoms in the five-membered ring are coplanar.
- The carbon–oxygen bond is drawn as a double bond. If the nitrogen and the carbon attached to the C=O group each contribute one electron to the bonding, use both a Lewis electron structure and a hybrid orbital approach to explain the presence of the double bond.
- If sulfur and carbon each contribute one electron to nitrogen, how many lone pairs are present on the nitrogen?
- What is the geometry of the sulfur atom?
- The Lewis electron structure supports a single bond between the carbon and the nitrogen and a double bond between the carbon and the oxygen. In actuality, the C–O bond is longer than expected for a double bond, and the C–N bond is shorter. The nitrogen is also planar. Based on this information, what is the likely hybridization of the nitrogen? Using the concepts of molecular orbital theory, propose an explanation for this observation.
-
♦ Pheromones are chemical signals used for communication between members of the same species. For example, the bark beetle uses an aggregation pheromone to signal other bark beetles to congregate at a particular site in a tree. Bark beetle infestations can cause severe damage because the beetles carry a fungal infection that spreads rapidly and can kill the tree. One of the components of this aggregation pheromone has the following structure:
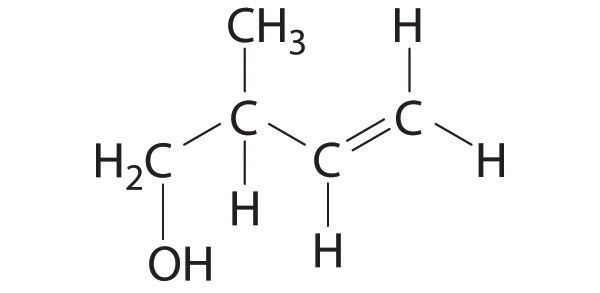
- Give the hybridization of all atoms except hydrogen in this pheromone.
- How many σ bonds are present in this molecule? How many π bonds are there?
- Describe the bonding in this molecule using a combination of the localized and delocalized approaches.
-
Carbon monoxide is highly poisonous because it binds more strongly than O2 to the iron in red blood cells, which transport oxygen in the blood. Consequently, a victim of CO poisoning suffocates from a lack of oxygen. Draw a molecular orbital energy-level diagram for CO. What is the highest occupied molecular orbital? Are any of the molecular orbitals degenerate? If so, which ones?
Problems marked with a ♦ involve multiple concepts.
Answer
-
-
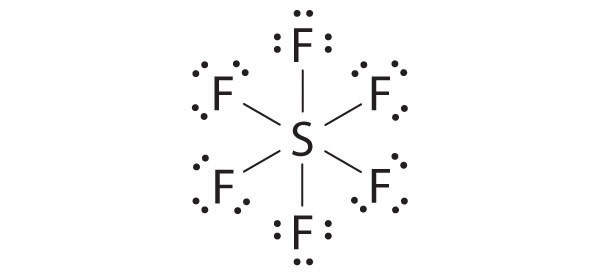
There are six electron groups, the molecular geometry is octahedral, and the hybridization of S is sp3d2.
- With six fluorine atoms packed around the central sulfur atom, there is no room for another species to approach the sulfur to initiate a reaction. The polar S–F bonds are also expected to be quite strong, so breaking an S–F bond to initiate a reaction is unlikely under most conditions.
- SF6 is a gas at room temperature because it has no net dipole moment; the individual S–F bond dipoles cancel one another in this highly symmetrical structure. The absence of a dipole moment results in very weak interactions between SF6 molecules, and as a result SF6 is a gas rather than a liquid or a solid at room temperature.
-
-
-
-
-




