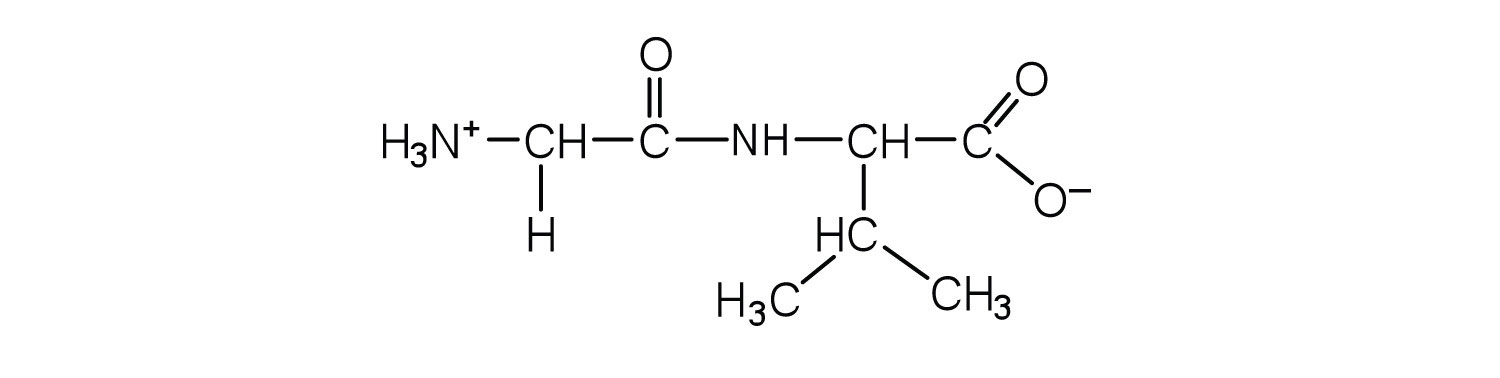
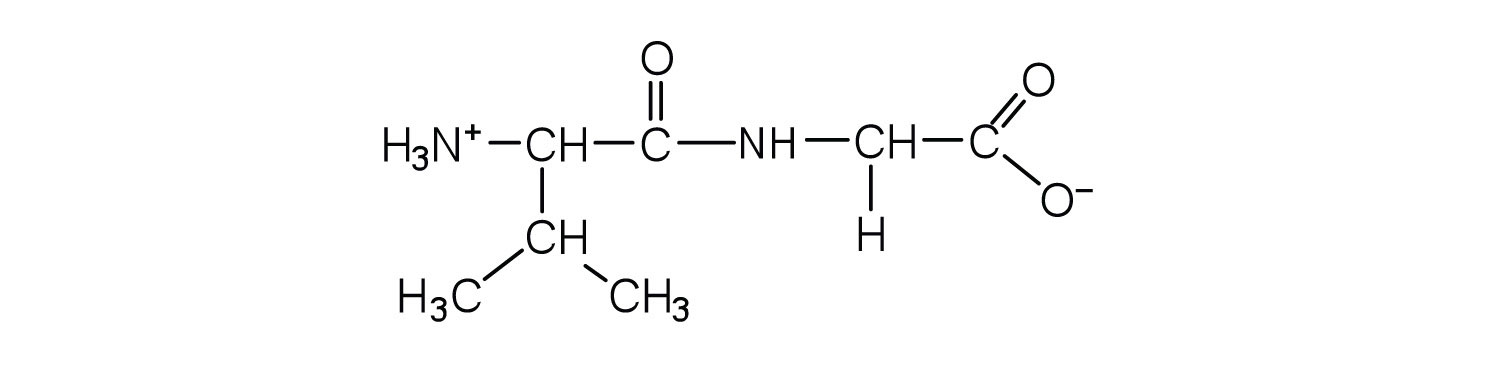
This is “Peptides”, section 18.3 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
18.3 Peptides
Learning Objectives
- Explain how a peptide is formed from individual amino acids.
- Explain why the sequence of amino acids in a protein is important.
Two or more amino acids can join together into chains called peptides. In Chapter 15 "Organic Acids and Bases and Some of Their Derivatives", Section 15.16 "Formation of Amides", we discussed the reaction between ammonia and a carboxylic acid to form an amide. In a similar reaction, the amino group on one amino acid molecule reacts with the carboxyl group on another, releasing a molecule of water and forming an amide linkage:
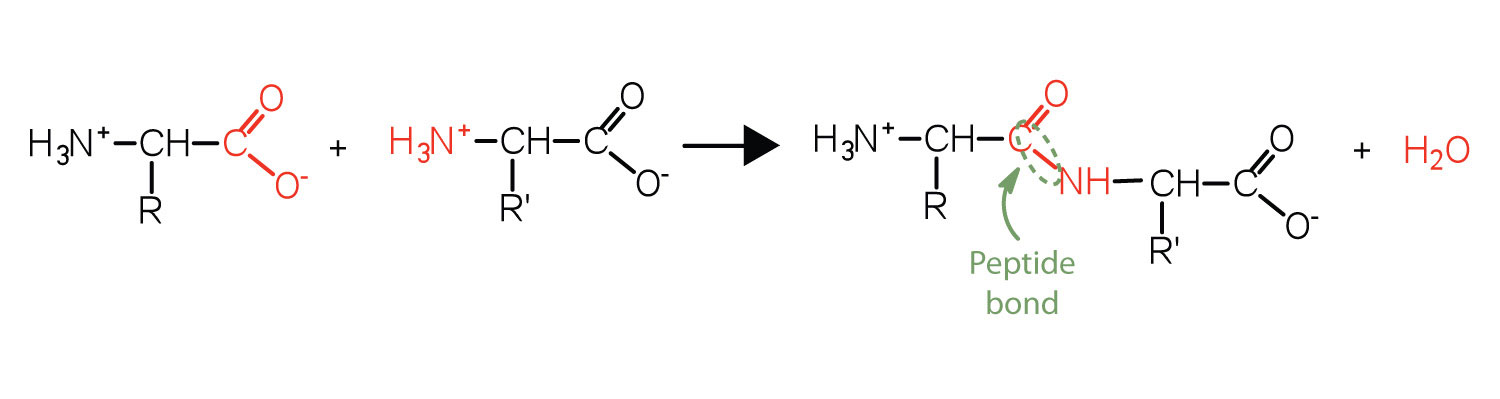
An amide bond joining two amino acid units is called a peptide bondThe amide bond joining two amino acid units in a peptide or protein.. Note that the product molecule still has a reactive amino group on the left and a reactive carboxyl group on the right. These can react with additional amino acids to lengthen the peptide. The process can continue until thousands of units have joined, resulting in large proteins.
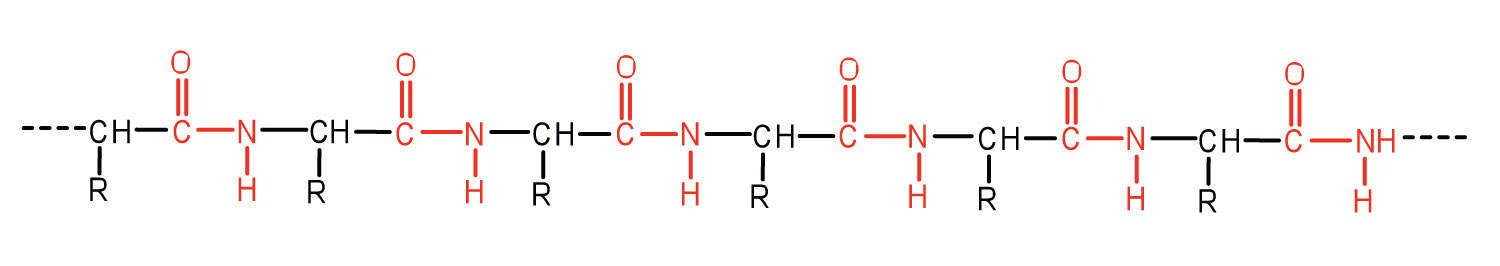
A chain consisting of only two amino acid units is called a dipeptide; a chain consisting of three is a tripeptide. By convention, peptide and protein structures are depicted with the amino acid whose amino group is free (the N-terminal end) on the left and the amino acid with a free carboxyl group (the C-terminal end) to the right.
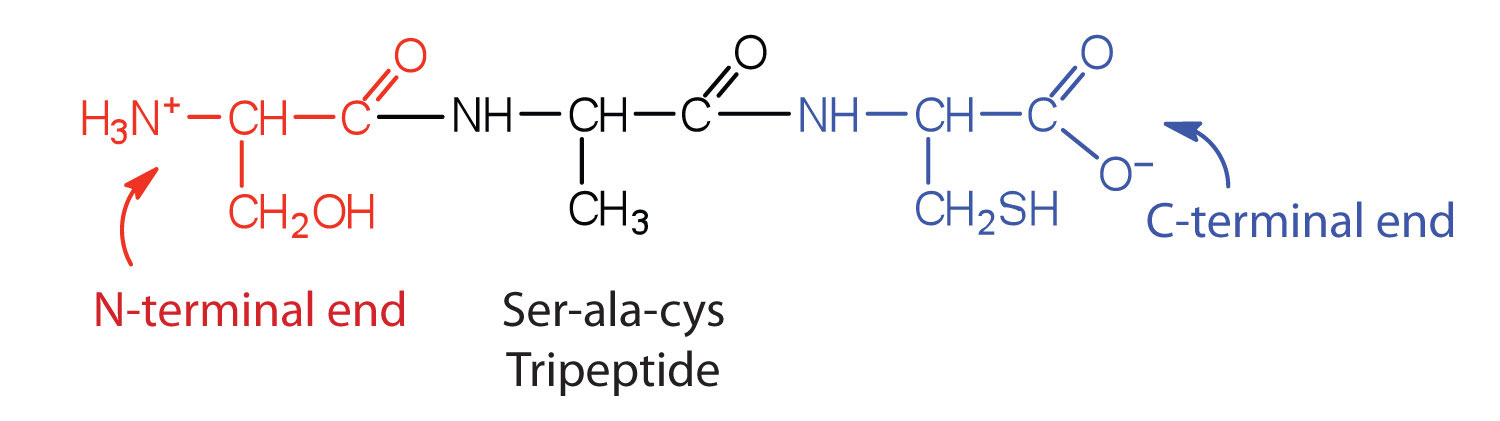
The general term peptide refers to an amino acid chain of unspecified length. However, chains of about 50 amino acids or more are usually called proteins or polypeptidesA chain of about 50 or more amino acids.. In its physiologically active form, a protein may be composed of one or more polypeptide chains.
For peptides and proteins to be physiologically active, it is not enough that they incorporate certain amounts of specific amino acids. The order, or sequence, in which the amino acids are connected is also of critical importance. Bradykinin is a nine-amino acid peptide produced in the blood that has the following amino acid sequence:
arg-pro-pro-gly-phe-ser-pro-phe-argThis peptide lowers blood pressure, stimulates smooth muscle tissue, increases capillary permeability, and causes pain. When the order of amino acids in bradykinin is reversed,
arg-phe-pro-ser-phe-gly-pro-pro-argthe peptide resulting from this synthesis shows none of the activity of bradykinin.
Just as millions of different words are spelled with our 26-letter English alphabet, millions of different proteins are made with the 20 common amino acids. However, just as the English alphabet can be used to write gibberish, amino acids can be put together in the wrong sequence to produce nonfunctional proteins. Although the correct sequence is ordinarily of utmost importance, it is not always absolutely required. Just as you can sometimes make sense of incorrectly spelled English words, a protein with a small percentage of “incorrect” amino acids may continue to function. However, it rarely functions as well as a protein having the correct sequence. There are also instances in which seemingly minor errors of sequence have disastrous effects. For example, in some people, every molecule of hemoglobin (a protein in the blood that transports oxygen) has a single incorrect amino acid unit out of about 300 (a single valine replaces a glutamic acid). That “minor” error is responsible for sickle cell anemia, an inherited condition that usually is fatal.
Concept Review Exercises
-
Distinguish between the N-terminal amino acid and the C-terminal amino acid of a peptide or protein.
-
Describe the difference between an amino acid and a peptide.
-
Amino acid units in a protein are connected by peptide bonds. What is another name for the functional group linking the amino acids?
Answers
-
The N-terminal end is the end of a peptide or protein whose amino group is free (not involved in the formation of a peptide bond), while the C-terminal end has a free carboxyl group.
-
A peptide is composed of two or more amino acids. Amino acids are the building blocks of peptides.
-
amide bond
Key Takeaways
- The amino group of one amino acid can react with the carboxyl group on another amino acid to form a peptide bond that links the two amino acids together. Additional amino acids can be added on through the formation of addition peptide (amide) bonds.
- A sequence of amino acids in a peptide or protein is written with the N-terminal amino acid first and the C-terminal amino acid at the end (writing left to right).
Exercises
-
Draw the structure for each peptide.
- gly-val
- val-gly
-
Draw the structure for cys-val-ala.
-
Identify the C- and N-terminal amino acids for the peptide lys-val-phe-gly-arg-cys.
-
Identify the C- and N-terminal amino acids for the peptide asp-arg-val-tyr-ile-his-pro-phe.
Answers
-
-
-
-
C-terminal amino acid: cys; N-terminal amino acid: lys
-