This is “Esters of Phosphoric Acid”, section 15.10 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
15.10 Esters of Phosphoric Acid
Learning Objectives
- Describe phosphate esters.
- Understand why phosphate esters are important in living cells.
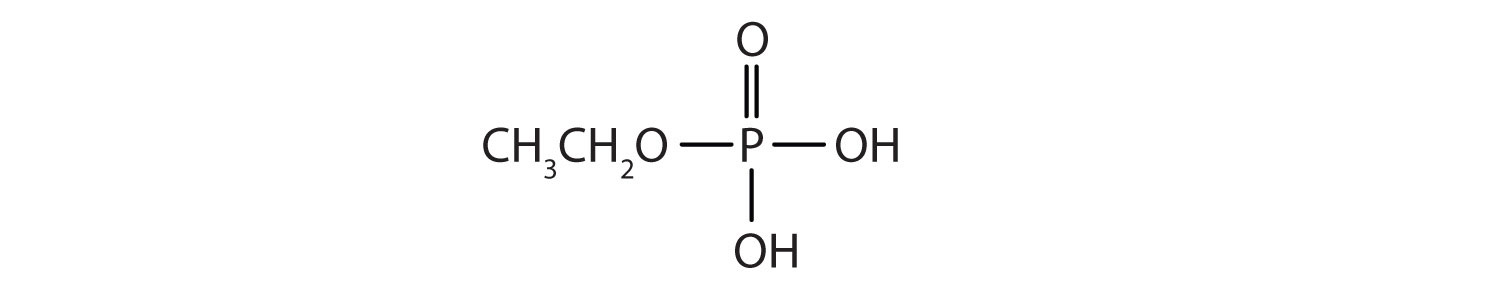
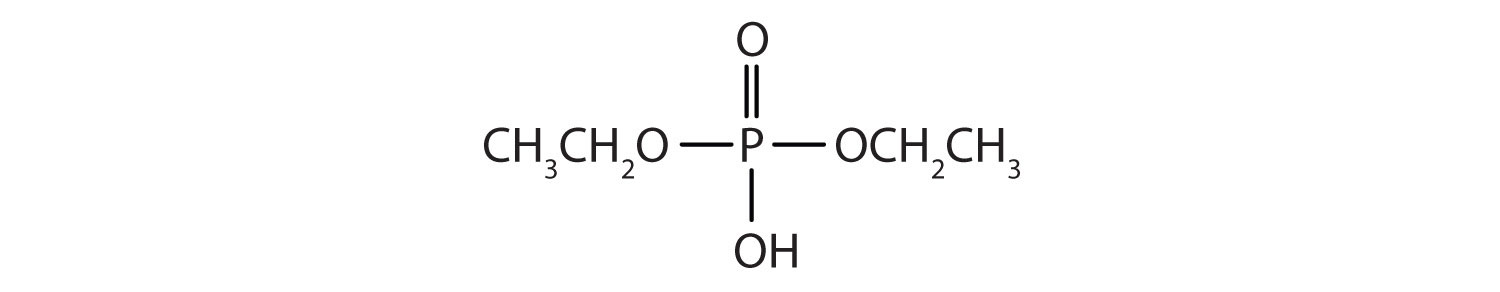
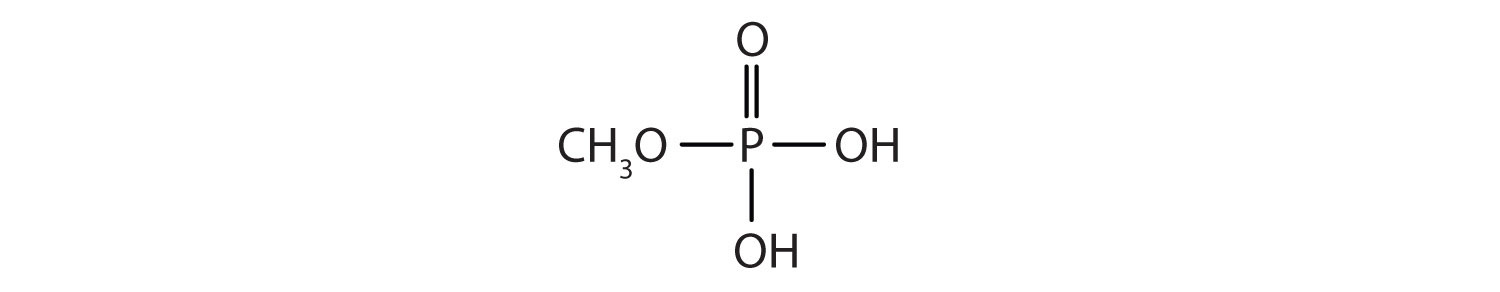
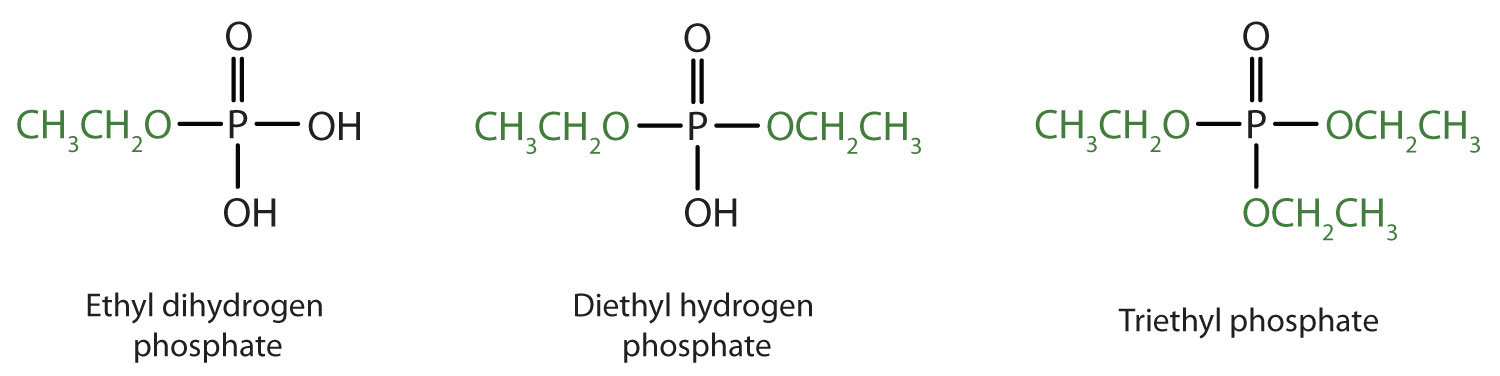
Just as carboxylic acids do, inorganic acids such as nitric acid (HNO3), sulfuric acid (H2SO4), and phosphoric acid (H3PO4) also form esters. The esters of phosphoric acid are especially important in biochemistry. A phosphoric acid molecule can form a monoalkyl, a dialkyl, or a trialkyl ester by reaction with one, two, or three molecules of an alcohol.
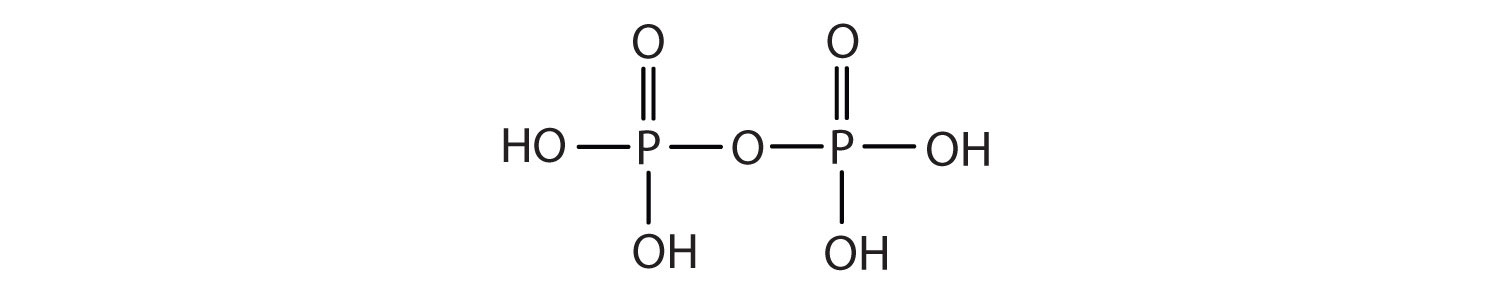
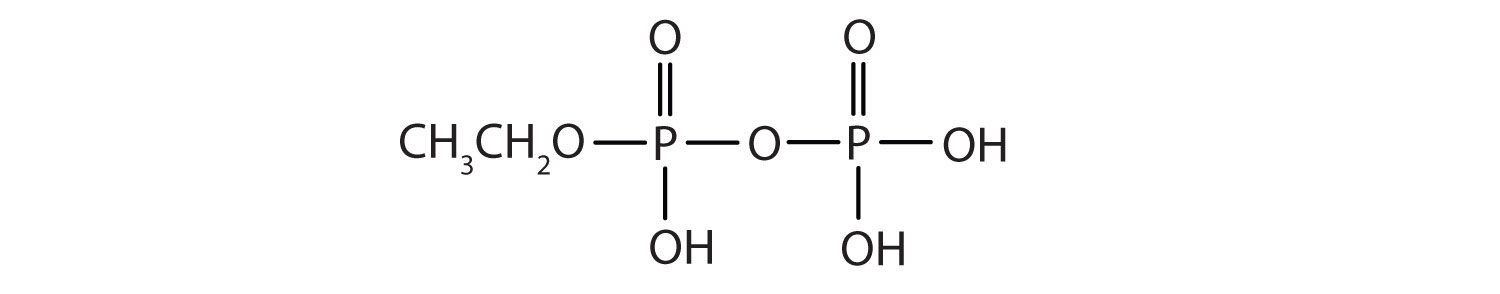
Esters of pyrophosphoric acid and triphosphoric acid are also important in biochemistry.
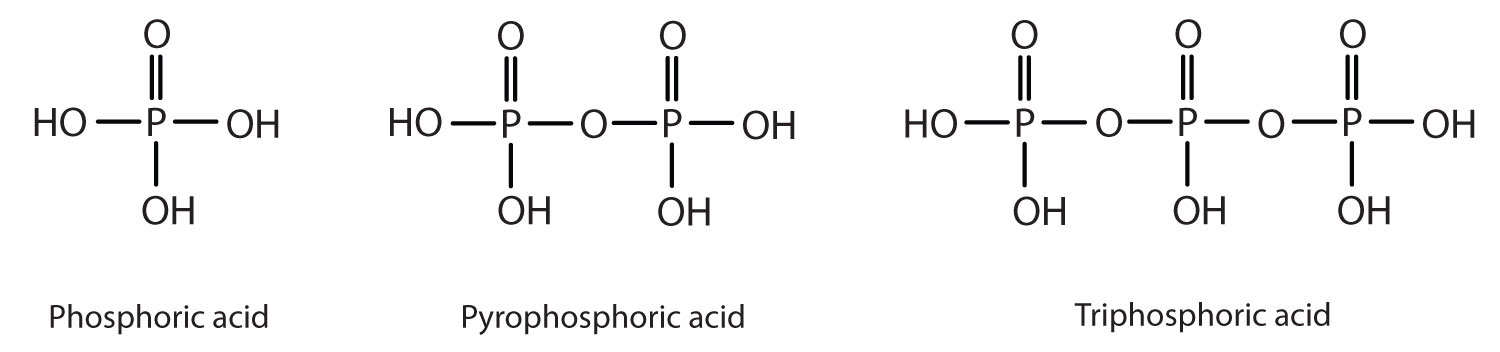
Esters of these acids are present in every plant and animal cell. They are biochemical intermediates in the transformation of food into usable energy. The bonds between phosphate units in adenosine triphosphate (ATP) are called phosphoanhydride bonds. These are high-energy bonds that store energy from the metabolism of foods. Hydrolysis of ATP releases energy as it is needed for biochemical processes (for instance, for muscle contraction). Phosphate esters are also important structural constituents of phospholipids and nucleic acids. (For more information about phospholipids and nucleic acids, see Chapter 17 "Lipids", Section 17.3 "Membranes and Membrane Lipids", and Chapter 19 "Nucleic Acids", respectively.)
Note
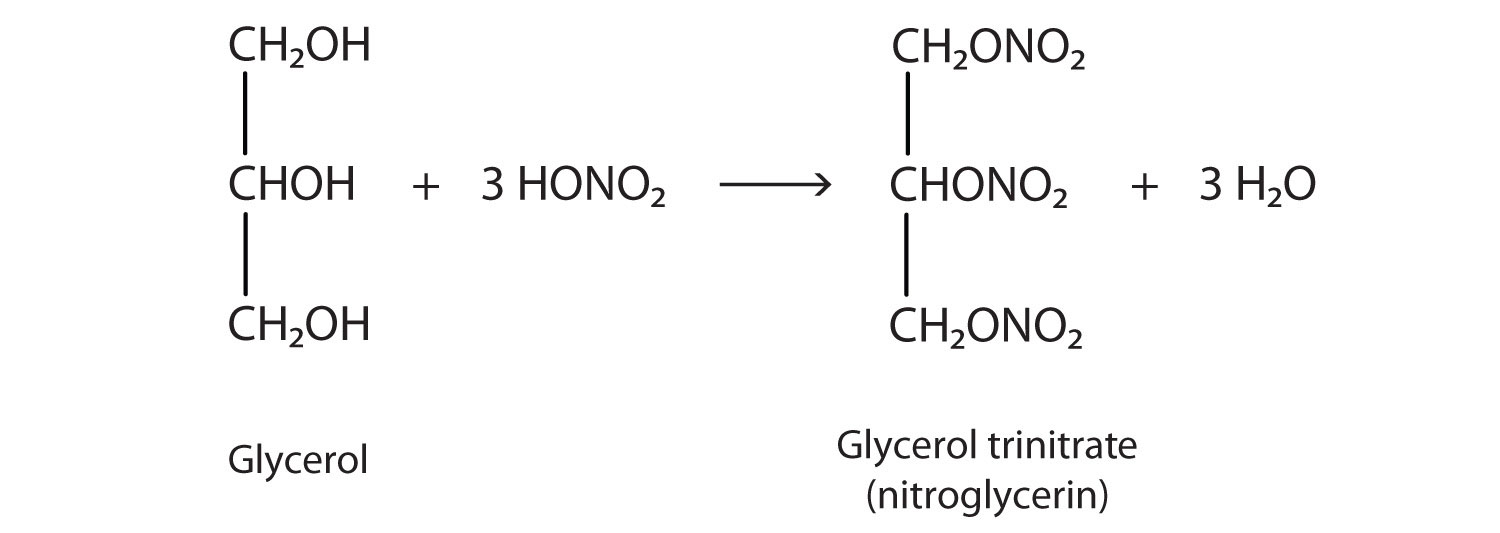
The explosive nitroglycerin (glyceryl trinitrate) is an ester formed from glycerol and nitric acid. It is used in medicine to relieve chest pain in heart disease.
Concept Review Exercise
-
What compounds combine to form phosphate esters?
Answer
-
phosphoric acids and alcohols
Key Takeaways
- Inorganic acids such as H3PO4 form esters.
- The esters of phosphoric acid are especially important in biochemistry.
Exercises
-
Draw the structure for each compound.
- diethyl hydrogen phosphate
- methyl dihydrogen phosphate
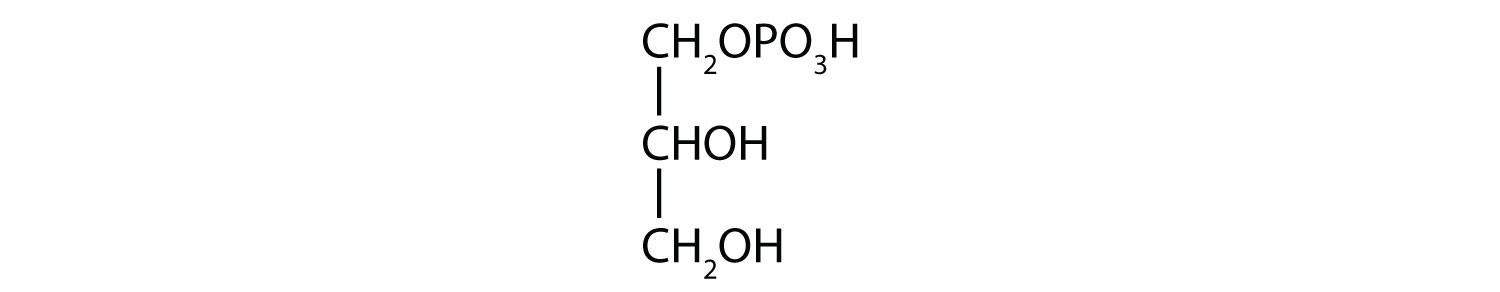
- 1-glycerol phosphate
-
Name each compound.
-