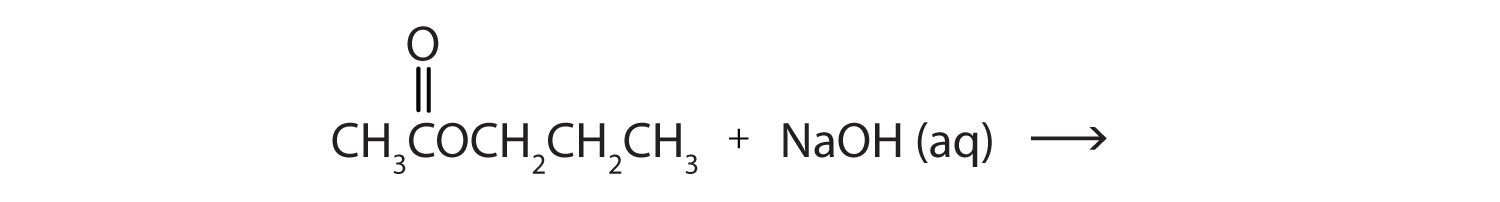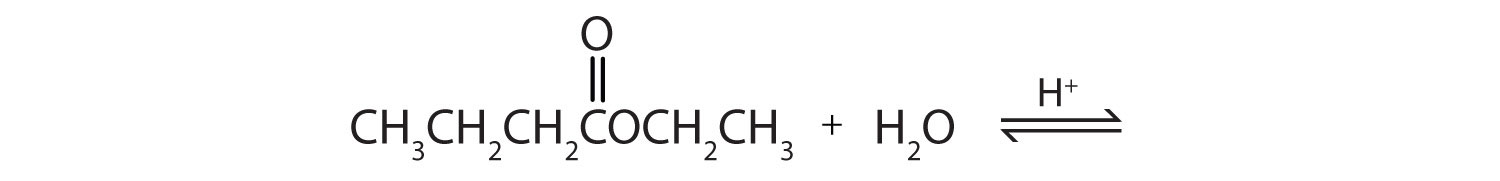This is “Hydrolysis of Esters”, section 15.9 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
15.9 Hydrolysis of Esters
Learning Objectives
- Describe the typical reaction that takes place with esters.
- Identify the products of an acidic hydrolysis of an ester.
- Identify the products of a basic hydrolysis of an ester.
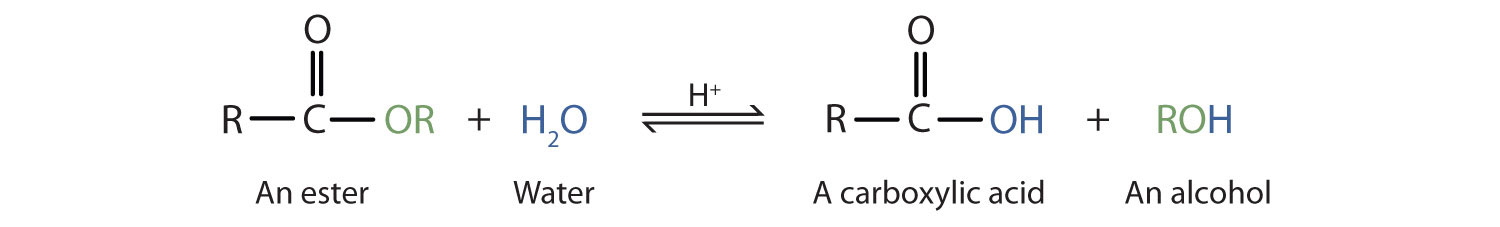
Esters are neutral compounds, unlike the acids from which they are formed. In typical reactions, the alkoxy (OR′) group of an ester is replaced by another group. One such reaction is hydrolysisThe reaction of a substance with water., literally “splitting with water.” The hydrolysis of esters is catalyzed by either an acid or a base.
Acidic hydrolysis is simply the reverse of esterification. The ester is heated with a large excess of water containing a strong-acid catalyst. Like esterification, the reaction is reversible and does not go to completion.
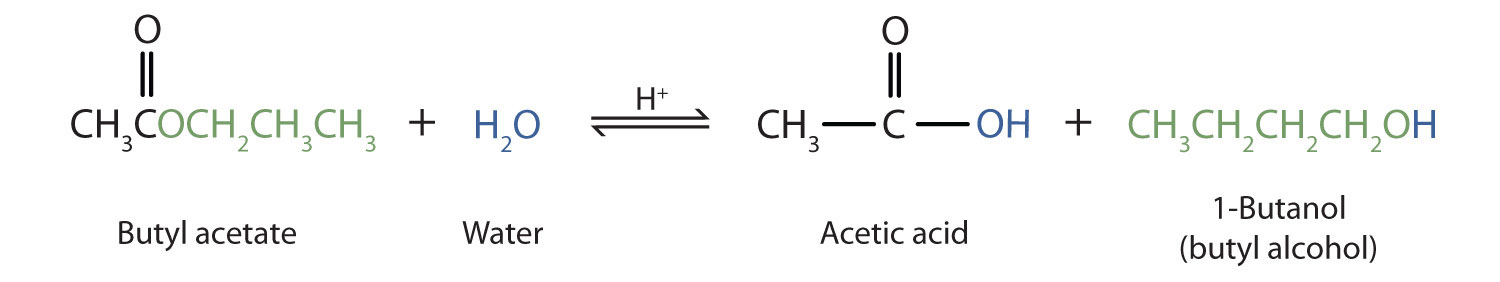
As a specific example, butyl acetate and water react to form acetic acid and 1-butanol. The reaction is reversible and does not go to completion.
Example 7
Write an equation for the acidic hydrolysis of ethyl butyrate (CH3CH2CH2COOCH2CH3) and name the products.
Solution
Remember that in acidic hydrolysis, water (HOH) splits the ester bond. The H of HOH joins to the oxygen atom in the OR part of the original ester, and the OH of HOH joins to the carbonyl carbon atom:
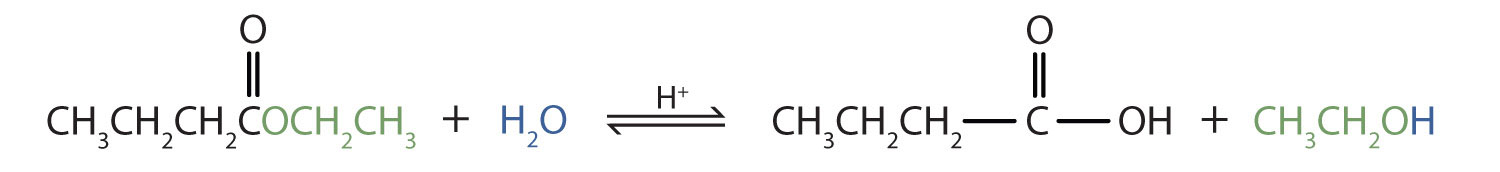
The products are butyric acid (butanoic acid) and ethanol.
Skill-Building Exercise
-
Write an equation for the acidic hydrolysis of methyl butanoate and name the products.
When a base (such as sodium hydroxide [NaOH] or potassium hydroxide [KOH]) is used to hydrolyze an ester, the products are a carboxylate salt and an alcohol. Because soaps are prepared by the alkaline hydrolysis of fats and oils, alkaline hydrolysis of esters is called saponificationThe hydrolysis of fats and oils in the presence of a base to make soap. (Latin sapon, meaning “soap,” and facere, meaning “to make”). In a saponification reaction, the base is a reactant, not simply a catalyst. The reaction goes to completion:
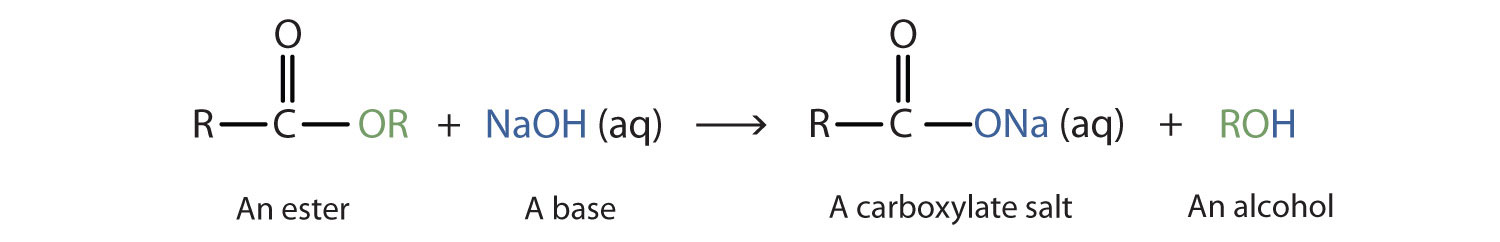
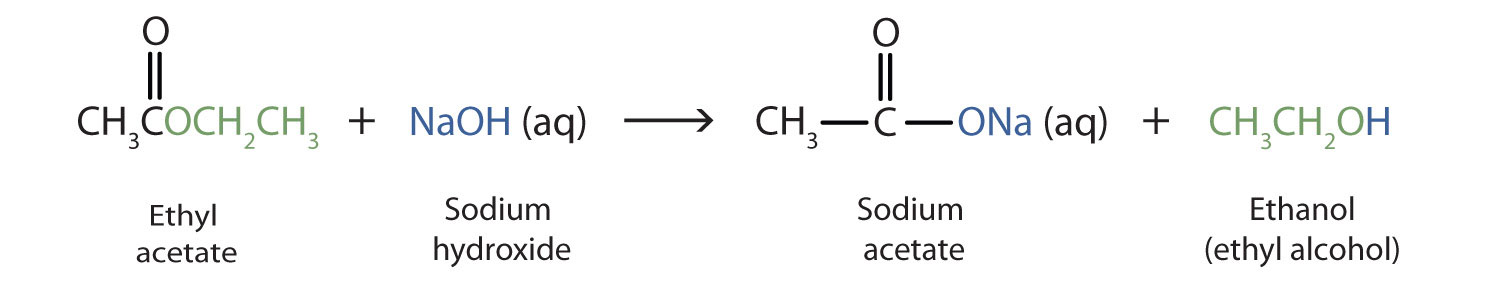
As a specific example, ethyl acetate and NaOH react to form sodium acetate and ethanol:
Example 8
Write an equation for the hydrolysis of methyl benzoate in a potassium hydroxide solution.
Solution
In basic hydrolysis, the molecule of the base splits the ester linkage. The acid portion of the ester ends up as the salt of the acid (in this case, the potassium salt). The alcohol portion of the ester ends up as the free alcohol.
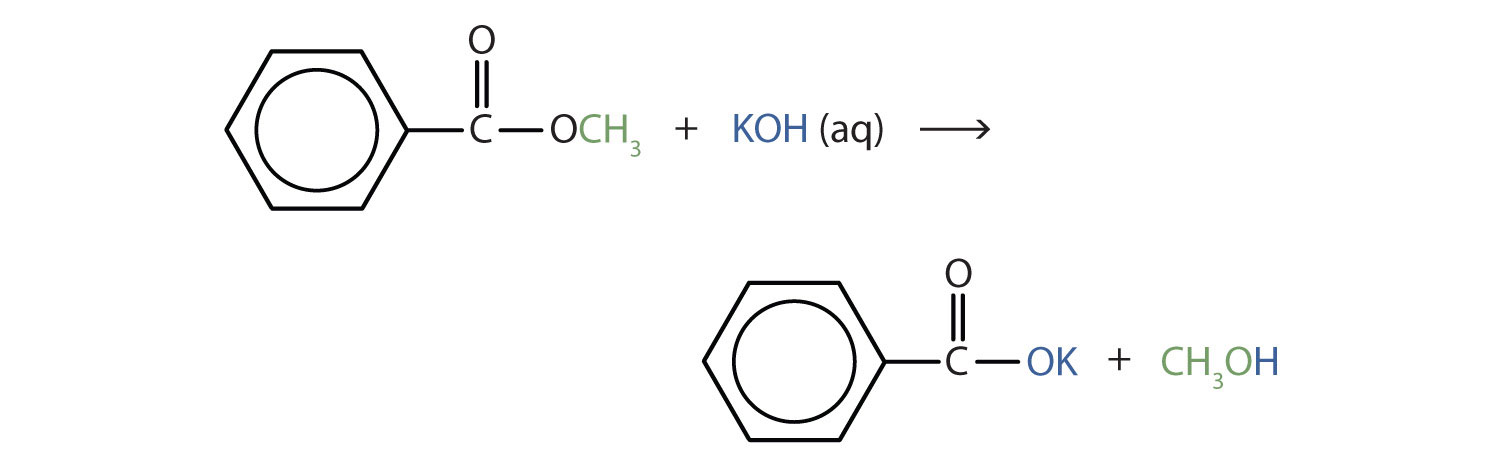
Skill-Building Exercise
-
Write the equation for the hydrolysis of ethyl propanoate in a sodium hydroxide solution.
Concept Review Exercises
-
How do acidic hydrolysis and basic hydrolysis of an ester differ in terms of
- products obtained?
- the extent of reaction?
-
What is saponification?
Answers
-
- acidic hydrolysis: carboxylic acid + alcohol; basic hydrolysis: carboxylate salt + alcohol
- basic hydrolysis: completion; acidic hydrolysis: incomplete reaction
-
the basic hydrolysis of an ester
Key Takeaways
- Hydrolysis is a most important reaction of esters.
- Acidic hydrolysis of an ester gives a carboxylic acid and an alcohol.
- Basic hydrolysis of an ester gives a carboxylate salt and an alcohol.
Exercises
-
Write an equation for the acid-catalyzed hydrolysis of ethyl acetate.
-
Write an equation for the base-catalyzed hydrolysis of ethyl acetate.
-
Complete each equation.
-
-
Complete each equation.
- CH3COOCH(CH3)2 + KOH(aq) →
Answers
-
-
-
- CH3COONa(aq) + CH3CH2CH2OH
- CH3CH2CH2COOH + CH3CH2OH
-