This is “Structure and Nomenclature of Aromatic Compounds”, section 13.8 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
13.8 Structure and Nomenclature of Aromatic Compounds
Learning Objectives
- Recognize aromatic compounds from structural formulas.
- Name aromatic compounds given formulas.
- Write formulas for aromatic compounds given their names.
Historically, benzene-like substances were called aromatic hydrocarbons because they had distinctive aromas. Today, an aromatic compoundAny compound that contains a benzene ring or has certain benzene-like properties. is any compound that contains a benzene ring or has certain benzene-like properties (but not necessarily a strong aroma). You can recognize the aromatic compounds in this text by the presence of one or more benzene rings in their structure. Some representative aromatic compounds and their uses are listed in Table 13.3 "Some Representative Aromatic Compounds", where the benzene ring is represented as C6H5.
Table 13.3 Some Representative Aromatic Compounds
| Name | Structure | Typical Uses |
|---|---|---|
| aniline | C6H5–NH2 | starting material for the synthesis of dyes, drugs, resins, varnishes, perfumes; solvent; vulcanizing rubber |
| benzoic acid | C6H5–COOH | food preservative; starting material for the synthesis of dyes and other organic compounds; curing of tobacco |
| bromobenzene | C6H5–Br | starting material for the synthesis of many other aromatic compounds; solvent; motor oil additive |
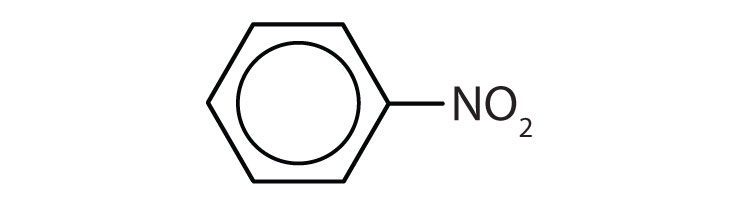
| nitrobenzene | C6H5–NO2 | starting material for the synthesis of aniline; solvent for cellulose nitrate; in soaps and shoe polish |
| phenol | C6H5–OH | disinfectant; starting material for the synthesis of resins, drugs, and other organic compounds |
| toluene | C6H5–CH3 | solvent; gasoline octane booster; starting material for the synthesis of benzoic acid, benzaldehyde, and many other organic compounds |
Example 5
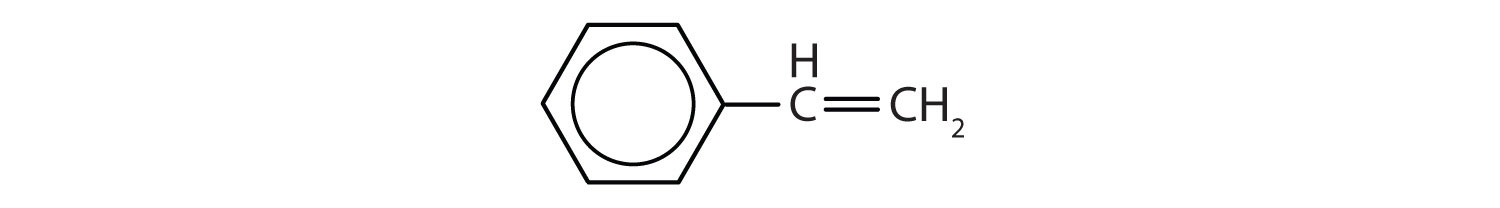
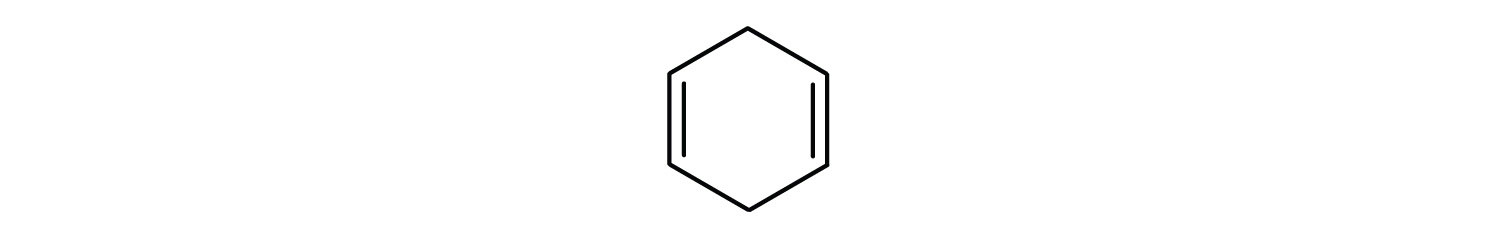
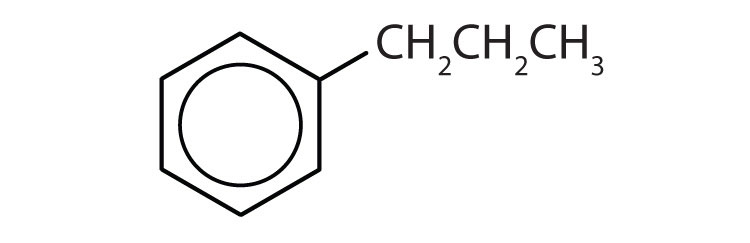
Which compounds are aromatic?
Solution
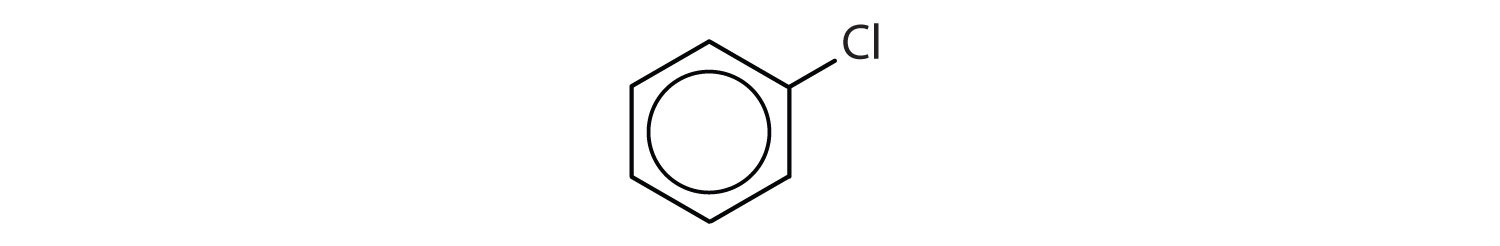
- The compound has a benzene ring (with a chlorine atom substituted for one of the hydrogen atoms); it is aromatic.
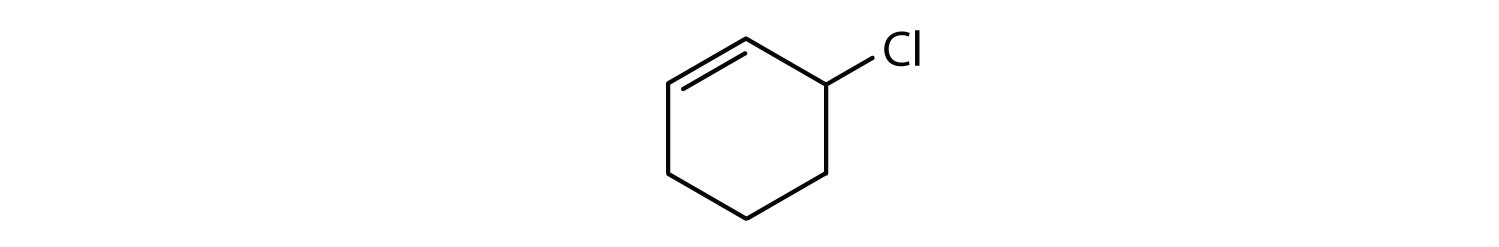
- The compound is cyclic, but it does not have a benzene ring; it is not aromatic.
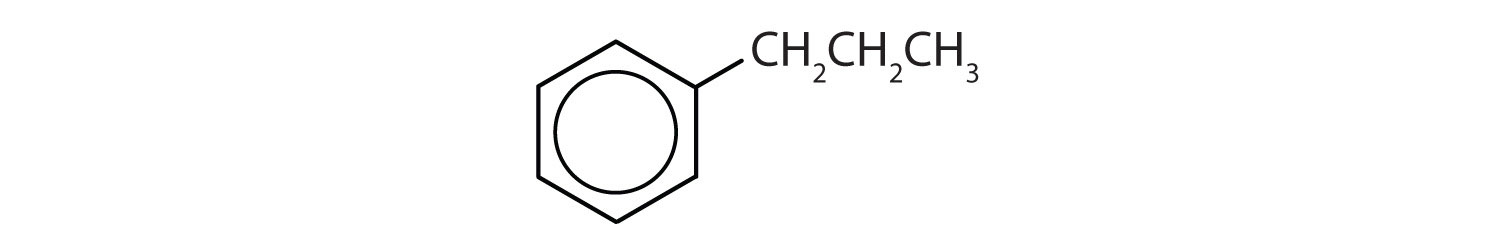
- The compound has a benzene ring (with a propyl group substituted for one of the hydrogen atoms); it is aromatic.
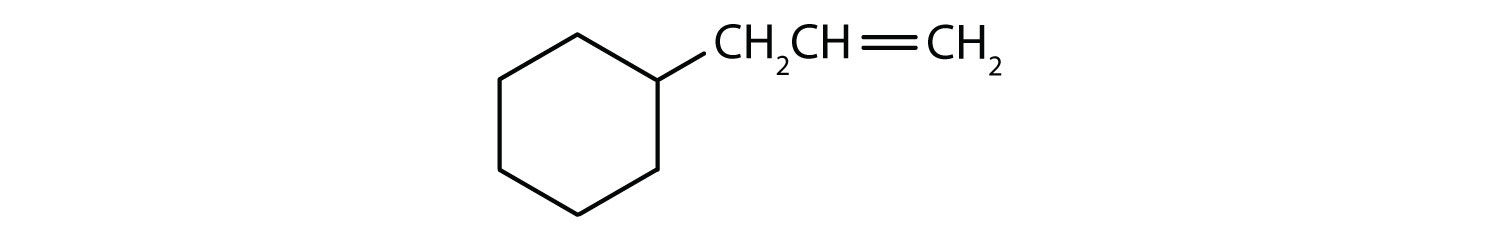
- The compound is cyclic, but it does not have a benzene ring; it is not aromatic.
Skill-Building Exercise
Which compounds are aromatic?
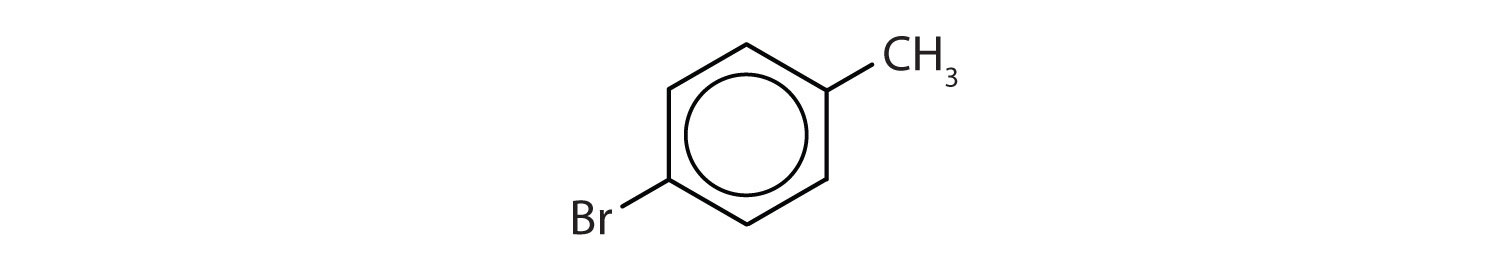
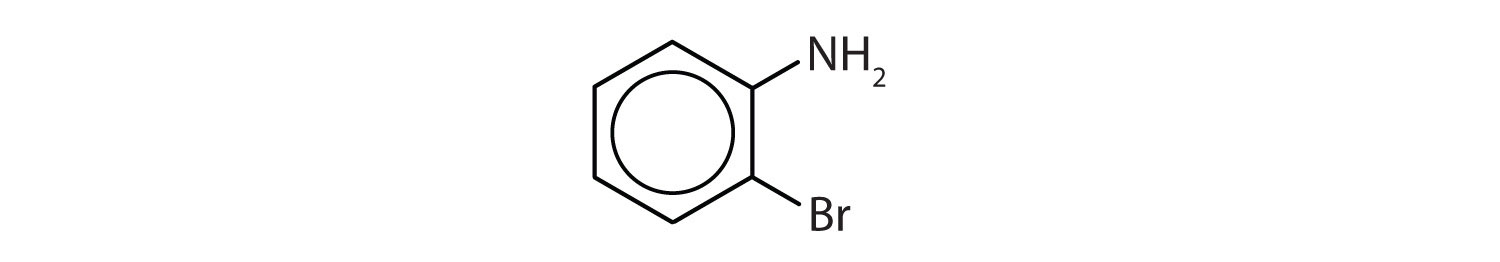
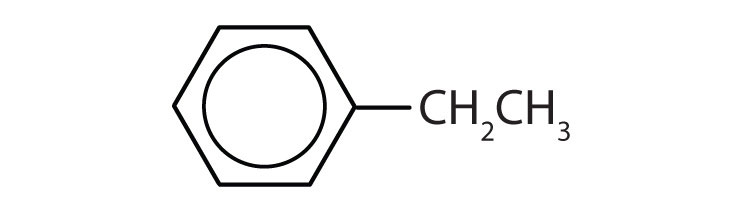
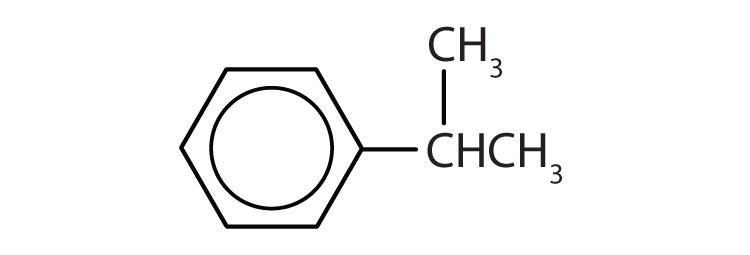
In the International Union of Pure and Applied Chemistry (IUPAC) system, aromatic hydrocarbons are named as derivatives of benzene. Figure 13.6 "Some Benzene Derivatives" shows four examples. In these structures, it is immaterial whether the single substituent is written at the top, side, or bottom of the ring: a hexagon is symmetrical, and therefore all positions are equivalent.
Figure 13.6 Some Benzene Derivatives
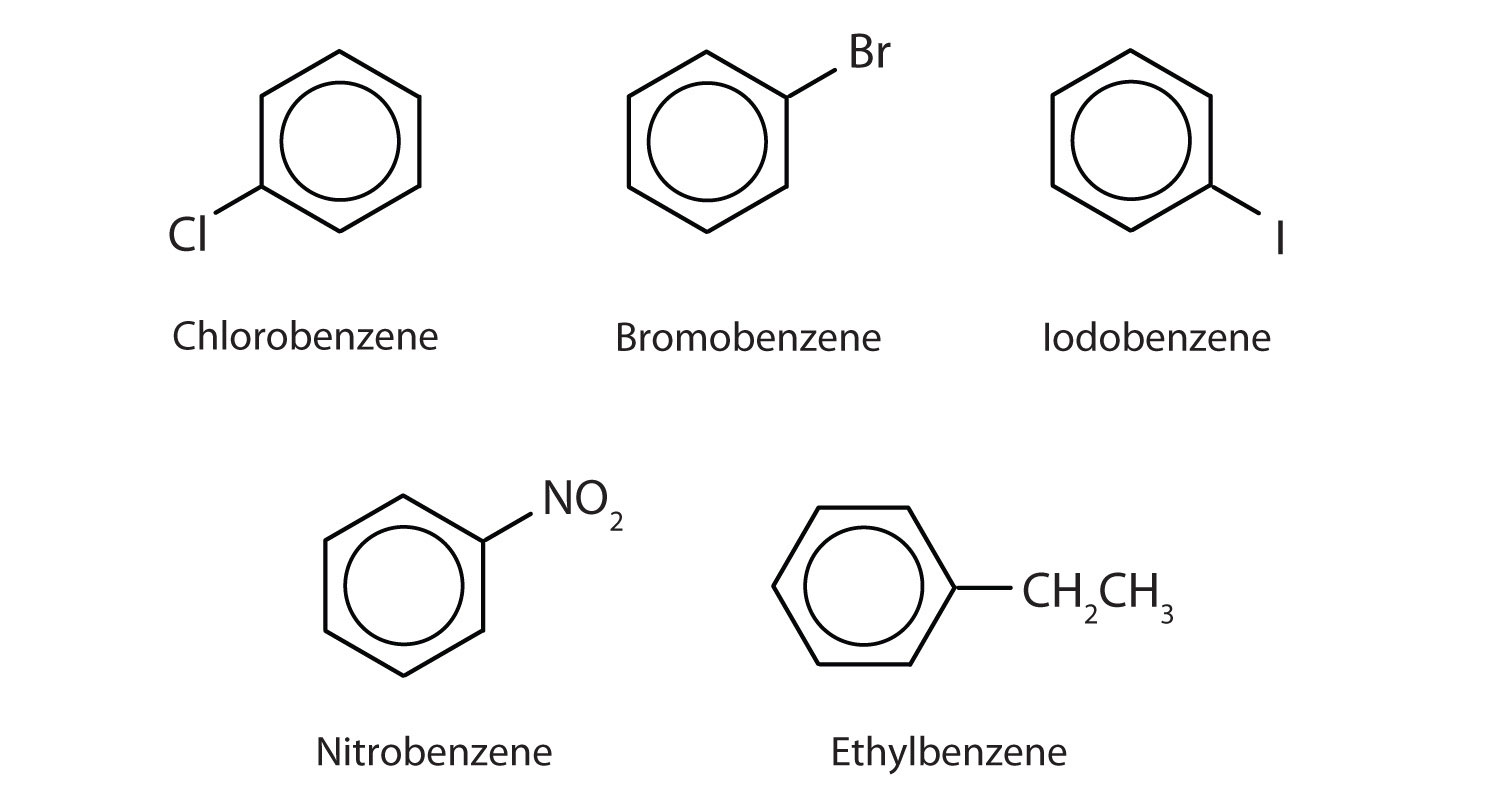
These compounds are named in the usual way with the group that replaces a hydrogen atom named as a substituent group: Cl as chloro, Br as bromo, I as iodo, NO2 as nitro, and CH3CH2 as ethyl.
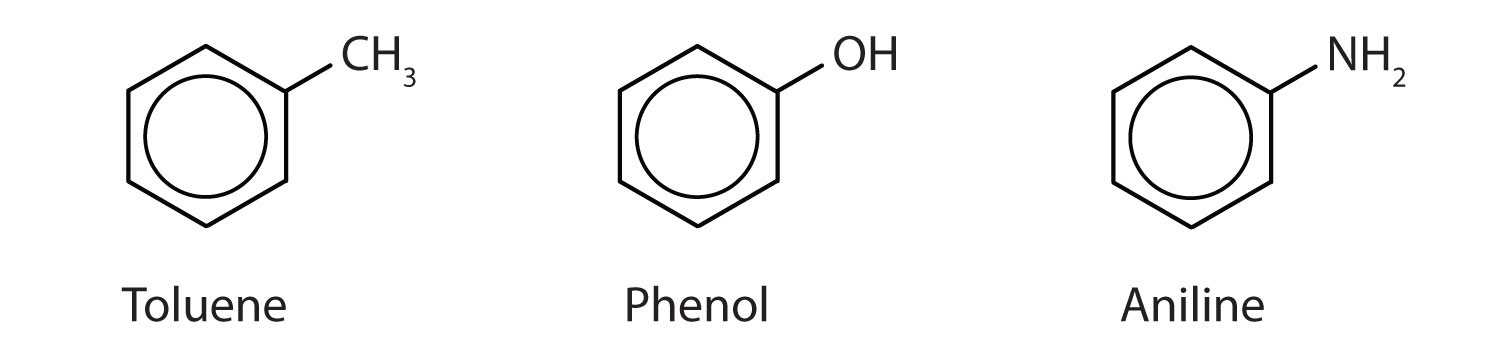
Although some compounds are referred to exclusively by IUPAC names, some are more frequently denoted by common names, as is indicated in Table 13.3 "Some Representative Aromatic Compounds".
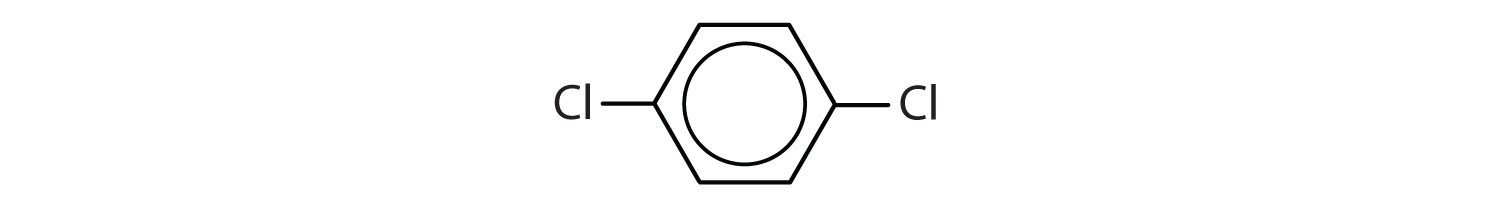
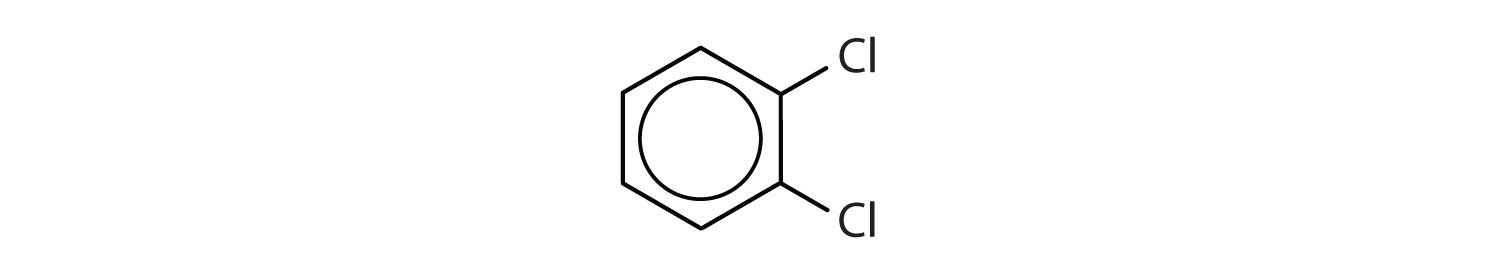
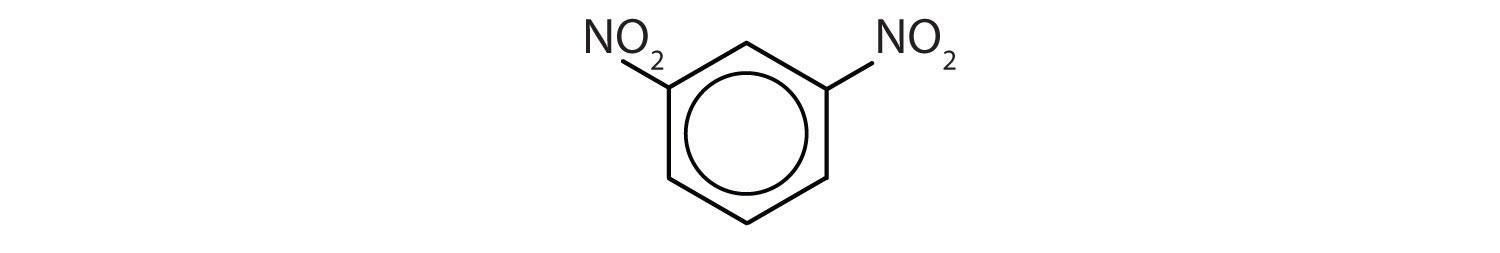
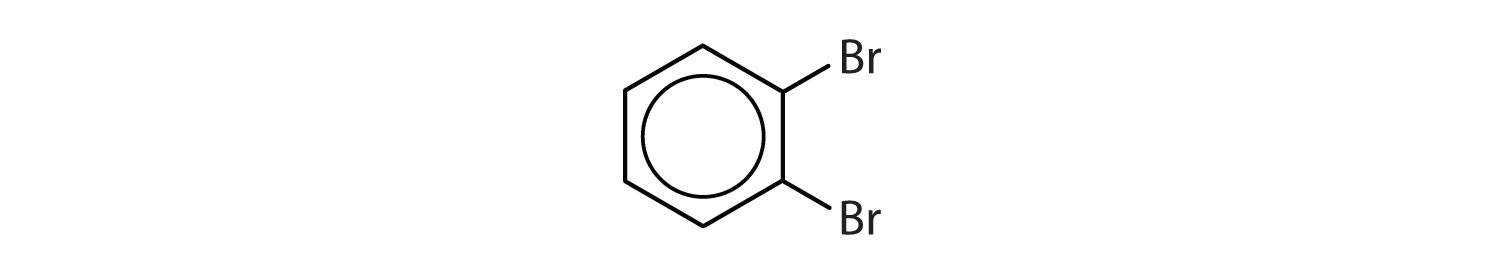
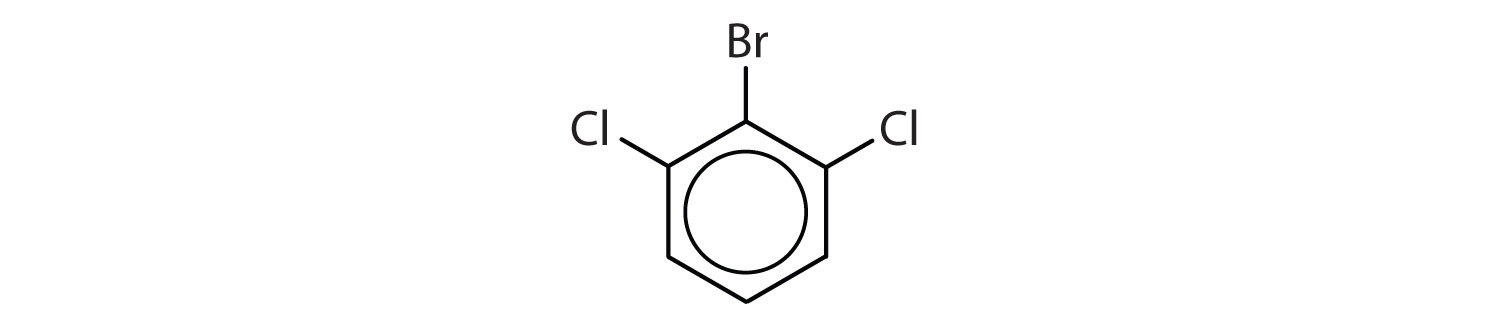
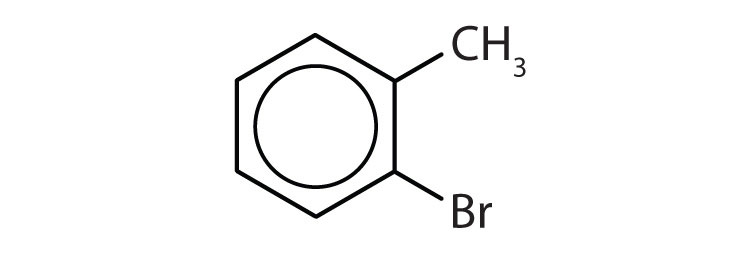
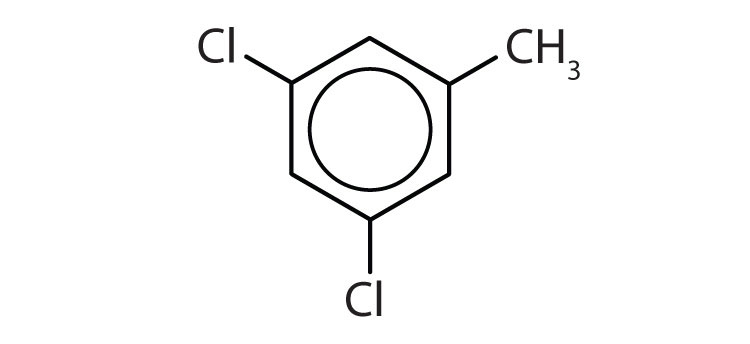
When there is more than one substituent, the corners of the hexagon are no longer equivalent, so we must designate the relative positions. There are three possible disubstituted benzenes, and we can use numbers to distinguish them (Figure 13.7 "The Three Isomeric Dichlorobenzenes"). We start numbering at the carbon atom to which one of the groups is attached and count toward the carbon atom that bears the other substituent group by the shortest path.
Figure 13.7 The Three Isomeric Dichlorobenzenes
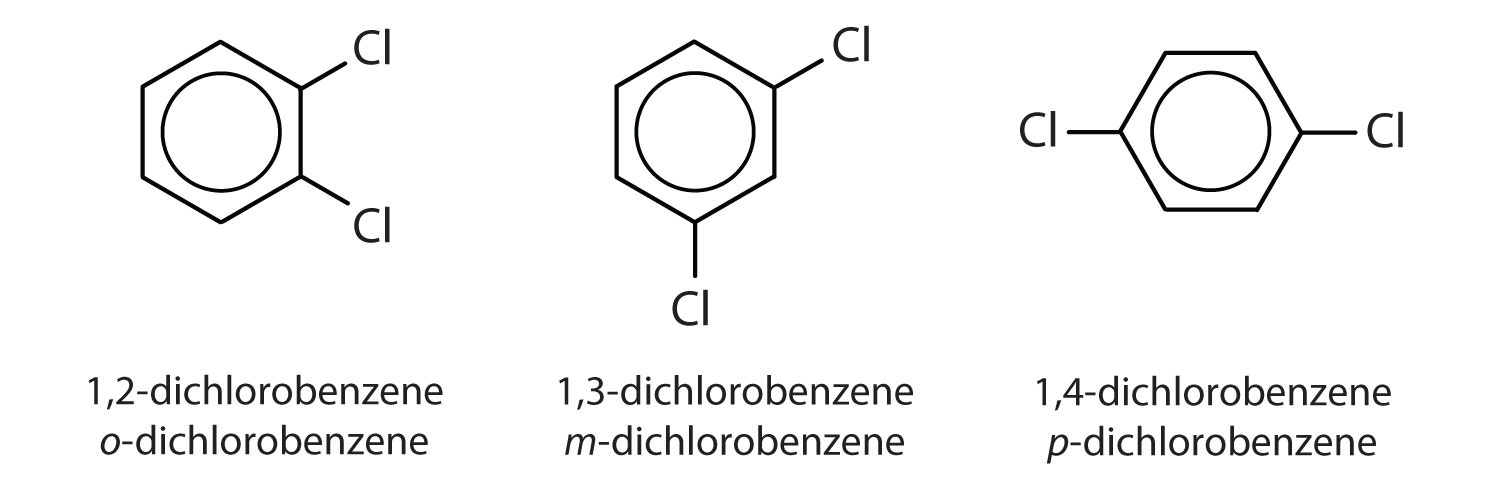
In Figure 13.7 "The Three Isomeric Dichlorobenzenes", common names are also used: the prefix ortho (o-) for 1,2-disubstitution, meta (m-) for 1,3-disubstitution, and para (p-) for 1,4-disubstitution.
The substituent names are listed in alphabetical order. The first substituent is given the lowest number. When a common name is used, the carbon atom that bears the group responsible for the name is given the number 1:
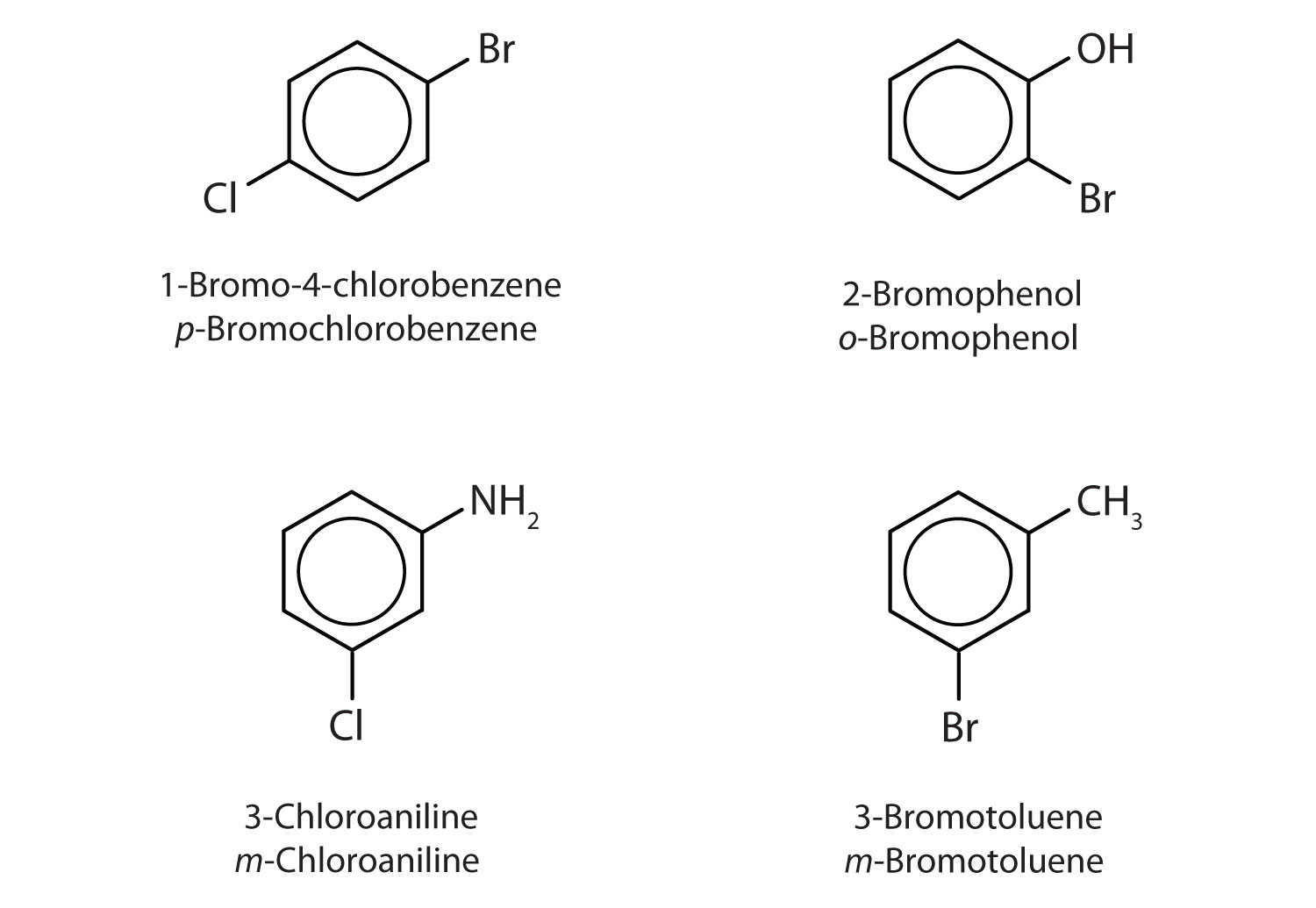
Example 6
Name each compound using both the common name and the IUPAC name.
Solution
- The benzene ring has two chlorine atoms (dichloro) in the first and second positions. The compound is o-dichlorobenzene or 1,2-dichlorobenzene.
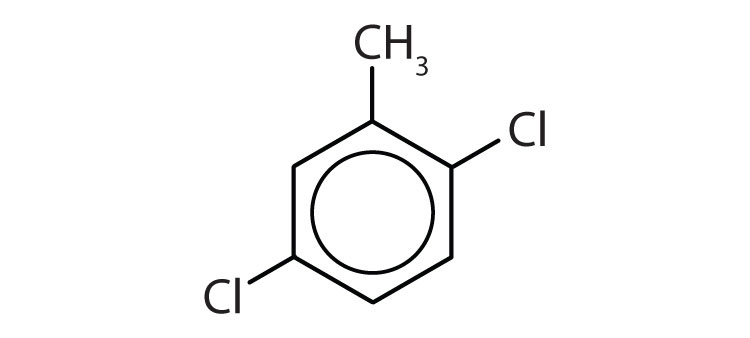
- The benzene ring has a methyl (CH3) group. The compound is therefore named as a derivative of toluene. The bromine atom is on the fourth carbon atom, counting from the methyl group. The compound is p-bromotoluene or 4-bromotoluene.
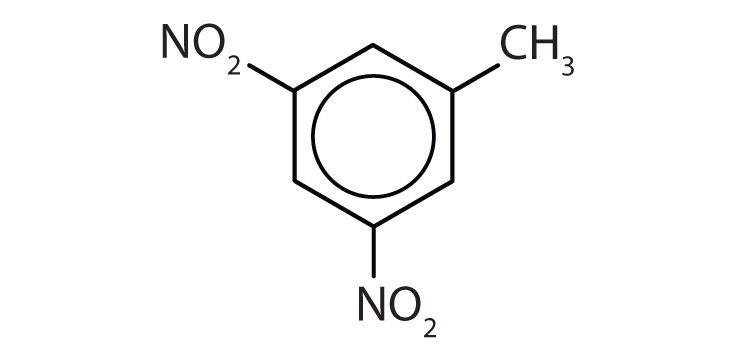
- The benzene ring has two nitro (NO2) groups in the first and third positions. It is m-dinitrobenzene or 1,3-dinitrobenzene.
Note
The nitro (NO2) group is a common substituent in aromatic compounds. Many nitro compounds are explosive, most notably 2,4,6-trinitrotoluene (TNT).
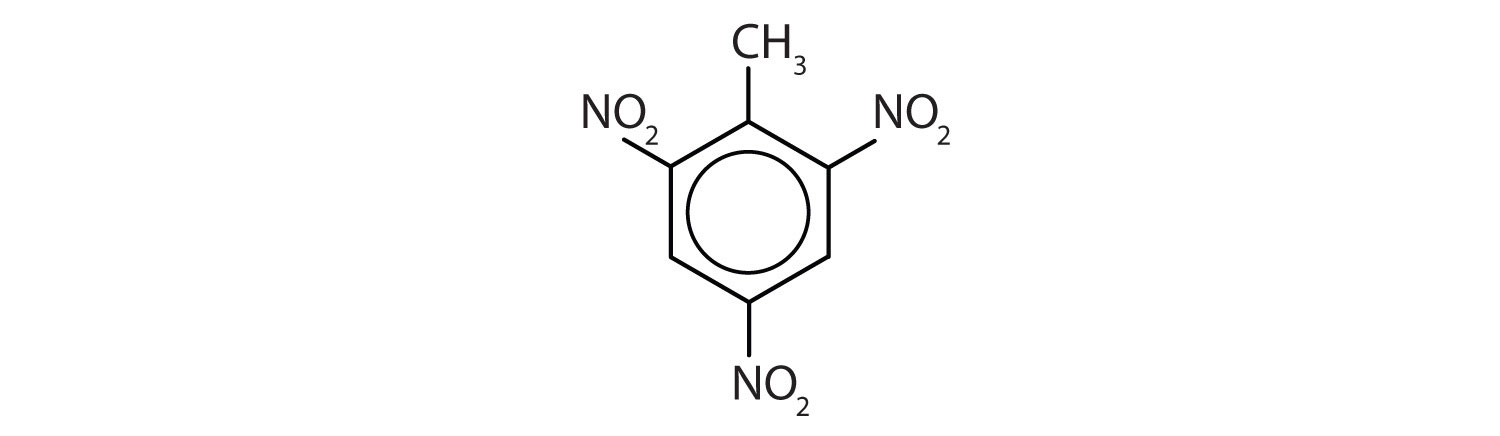
Skill-Building Exercise
Name each compound using both the common name and the IUPAC name.
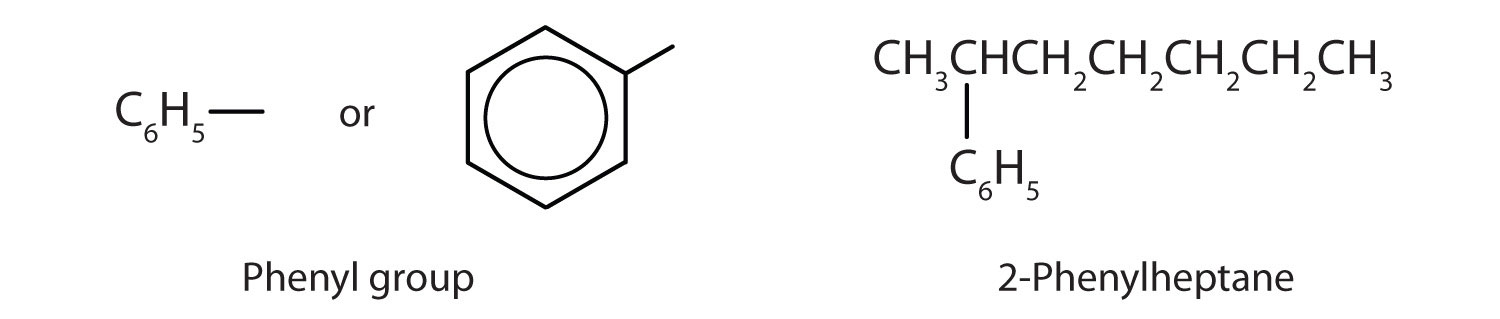
Sometimes an aromatic group is found as a substituent bonded to a nonaromatic entity or to another aromatic ring. The group of atoms remaining when a hydrogen atom is removed from an aromatic compound is called an aryl groupA group derived from an aromatic hydrocarbon by the removal of a hydrogen atom.. The most common aryl group is derived from benzene (C6H6) by removing one hydrogen atom (C6H5) and is called a phenyl group, from pheno, an old name for benzene.
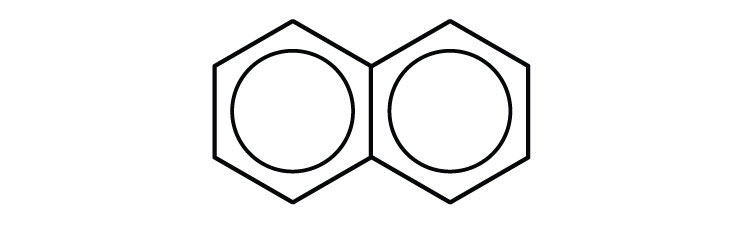
Polycyclic Aromatic Hydrocarbons
Some common aromatic hydrocarbons consist of fused benzene rings—rings that share a common side. These compounds are called polycyclic aromatic hydrocarbons (PAHs)An aromatic hydrocarbon consisting of fused benzene rings sharing a common side..
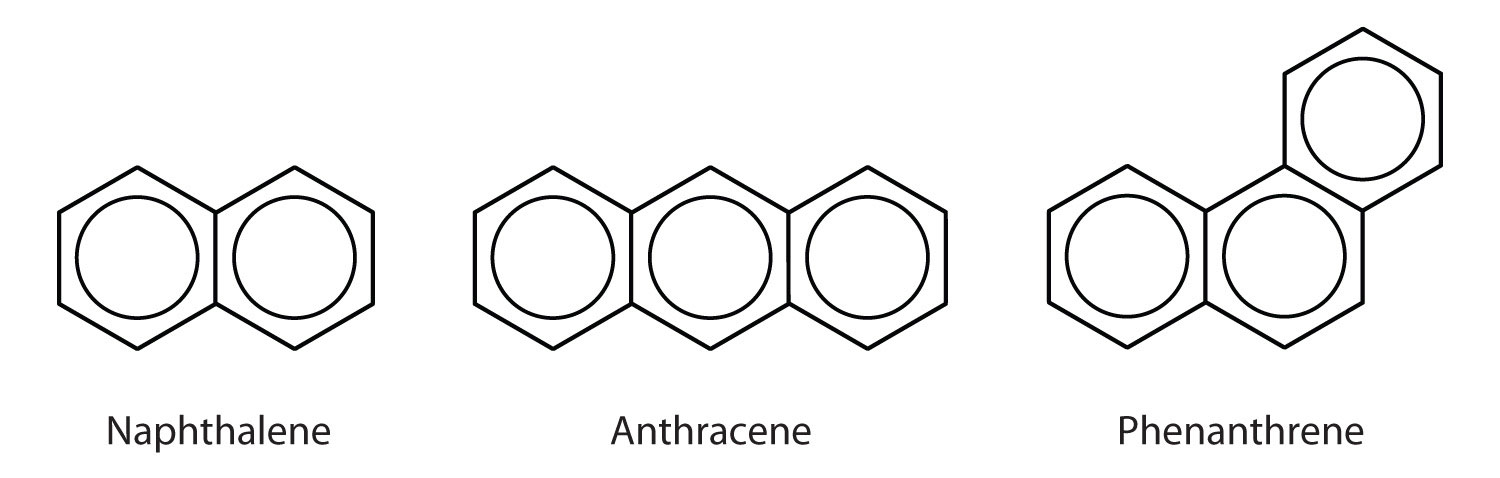
The three examples shown here are colorless, crystalline solids generally obtained from coal tar. Naphthalene has a pungent odor and is used in mothballs. Anthracene is used in the manufacture of certain dyes. Steroids, a large group of naturally occurring substances, contain the phenanthrene structure. (For more information about steroids, see Chapter 17 "Lipids", Section 17.4 "Steroids".)
To Your Health: Polycyclic Aromatic Hydrocarbons and Cancer
The intense heating required for distilling coal tar results in the formation of PAHs. For many years, it has been known that workers in coal-tar refineries are susceptible to a type of skin cancer known as tar cancer. Investigations have shown that a number of PAHs are carcinogens. One of the most active carcinogenic compounds, benzopyrene, occurs in coal tar and has also been isolated from cigarette smoke, automobile exhaust gases, and charcoal-broiled steaks. It is estimated that more than 1,000 t of benzopyrene are emitted into the air over the United States each year. Only a few milligrams of benzopyrene per kilogram of body weight are required to induce cancer in experimental animals.
Biologically Important Compounds with Benzene Rings
Substances containing the benzene ring are common in both animals and plants, although they are more abundant in the latter. Plants can synthesize the benzene ring from carbon dioxide, water, and inorganic materials. Animals cannot synthesize it, but they are dependent on certain aromatic compounds for survival and therefore must obtain them from food. Phenylalanine, tyrosine, and tryptophan (essential amino acids) and vitamins K, B2 (riboflavin), and B9 (folic acid) all contain the benzene ring. (For more information about vitamins, see Chapter 18 "Amino Acids, Proteins, and Enzymes", Section 18.9 "Enzyme Cofactors and Vitamins".) Many important drugs, a few of which are shown in Table 13.4 "Some Drugs That Contain a Benzene Ring", also feature a benzene ring.
Note
So far we have studied only aromatic compounds with carbon-containing rings. However, many cyclic compounds have an element other than carbon atoms in the ring. These compounds, called heterocyclic compounds, are discussed in Chapter 15 "Organic Acids and Bases and Some of Their Derivatives", Section 15.13 "Amines as Bases". Some of these are heterocyclic aromatic compounds.
Table 13.4 Some Drugs That Contain a Benzene Ring
| Name | Structure |
|---|---|
| aspirin |
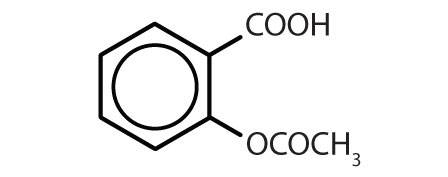
|
| acetaminophen |
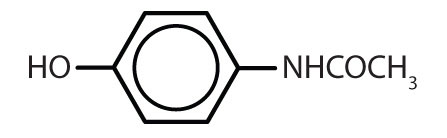
|
| ibuprofen |
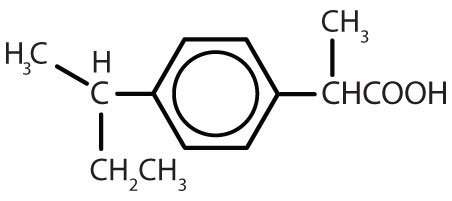
|
| amphetamine |
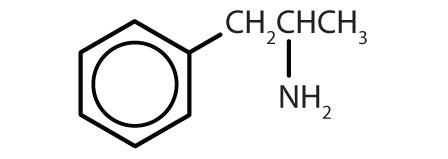
|
| sulfanilamide |
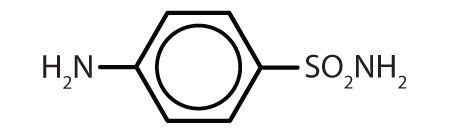
|
Concept Review Exercises
-
Briefly identify the important characteristics of an aromatic compound.
-
What is meant by the prefixes meta, ortho, or para? Give the name and draw the structure for a compound that illustrates each.
-
What is a phenyl group? Give the structure for 3-phenyloctane.
Answers
-
An aromatic compound is any compound that contains a benzene ring or has certain benzene-like properties.
-
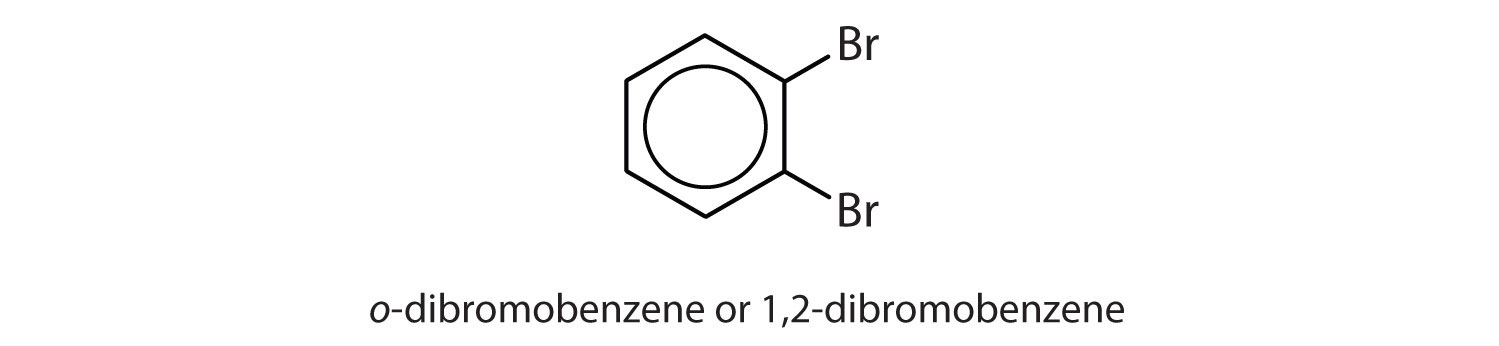
meta = 1,3 disubstitution; (answers will vary)
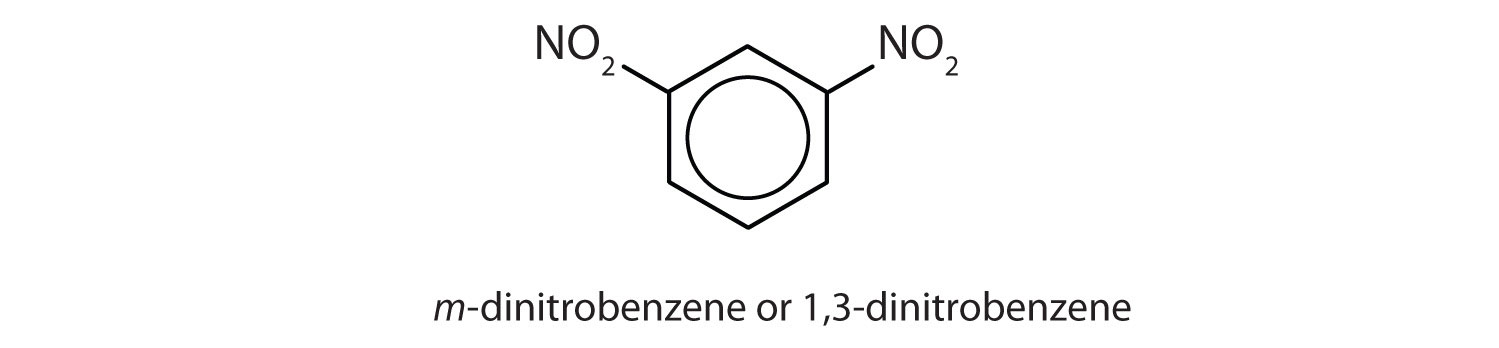
ortho = 1,2 disubstitution
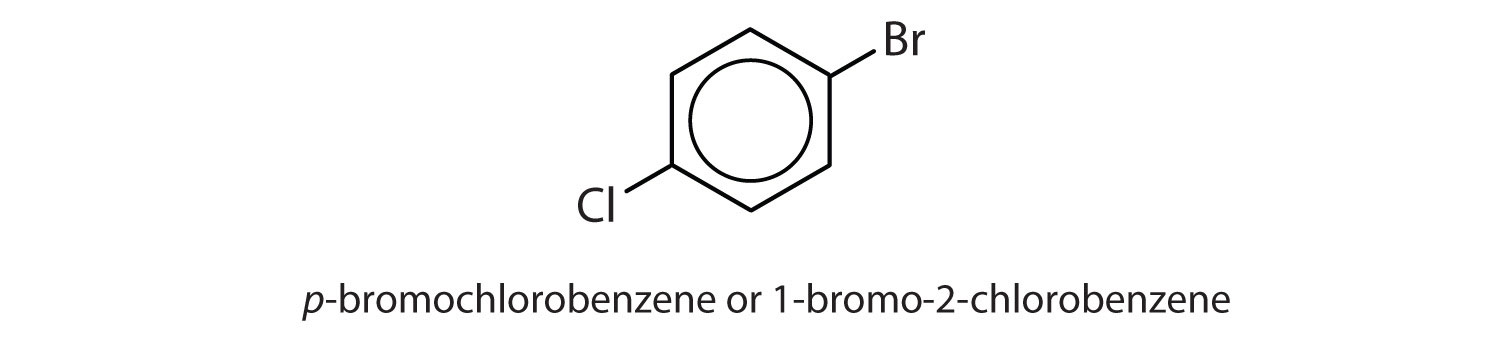
para = 1,4 disubstitution or 1-bromo-4-chlorobenzene
-
phenyl group: C6H5 or
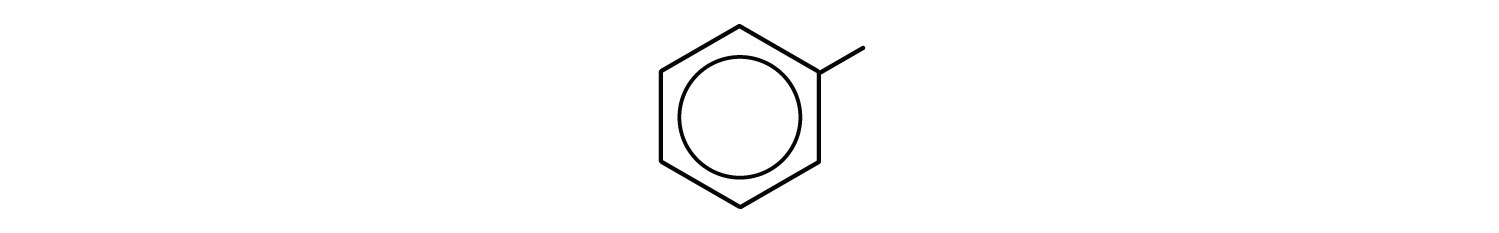
3-phenyloctane:
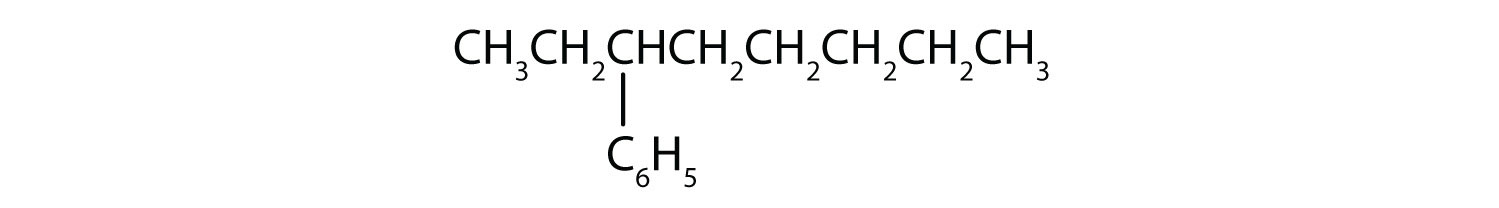
Key Takeaway
- Aromatic compounds contain a benzene ring or have certain benzene-like properties; for our purposes, you can recognize aromatic compounds by the presence of one or more benzene rings in their structure.
Exercises
-
Is each compound aromatic?
-
-
Is each compound aromatic?
-
-
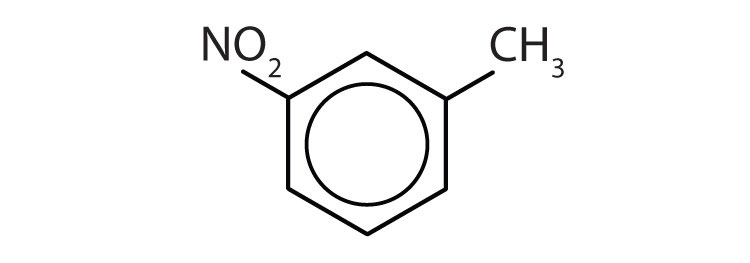
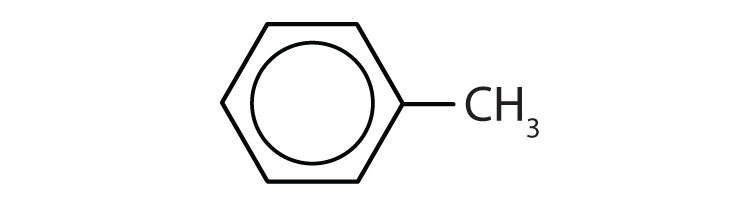
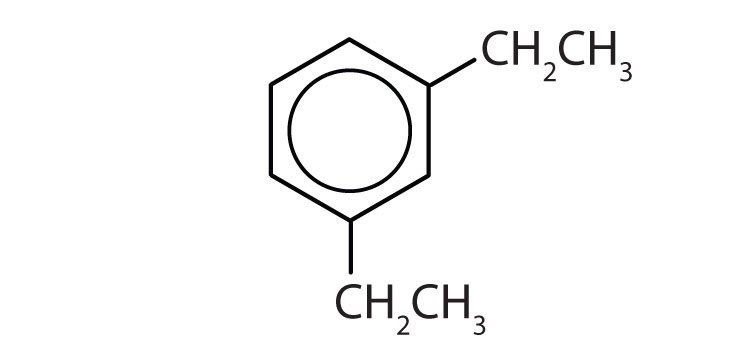
Draw the structure for each compound.
- toluene
- m-diethylbenzene
- 3,5-dinitrotoluene
-
Draw the structure for each compound.
- p-dichlorobenzene
- naphthalene
- 1,2,4-trimethylbenzene
-
Name each compound with its IUPAC name.
-
-
Name each compound with its IUPAC name.
-
Answers
-
- yes
- no
-
-
-
-
-
- ethylbenzene
- isopropylbenzene
- o-bromotoluene
- 3,5-dichlorotoluene
-