This is “The Elements of Group 14”, section 22.2 from the book Principles of General Chemistry (v. 1.0M). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
22.2 The Elements of Group 14
Learning Objective
- To understand the trends in properties and reactivity of the group 14 elements.
The elements of group 14 show a greater range of chemical behavior than any other family in the periodic table. Three of the five elements—carbon, tin, and lead—have been known since ancient times. For example, some of the oldest known writings are Egyptian hieroglyphics written on papyrus with ink made from lampblack, a finely divided carbon soot produced by the incomplete combustion of hydrocarbons (Figure 22.5 "Very Small Particles of Noncrystalline Carbon Are Used to Make Black Ink"). Activated carbon is an even more finely divided form of carbon that is produced from the thermal decomposition of organic materials, such as sawdust. Because it adsorbs many organic and sulfur-containing compounds, activated carbon is used to decolorize foods, such as sugar, and to purify gases and wastewater.
Figure 22.5 Very Small Particles of Noncrystalline Carbon Are Used to Make Black Ink
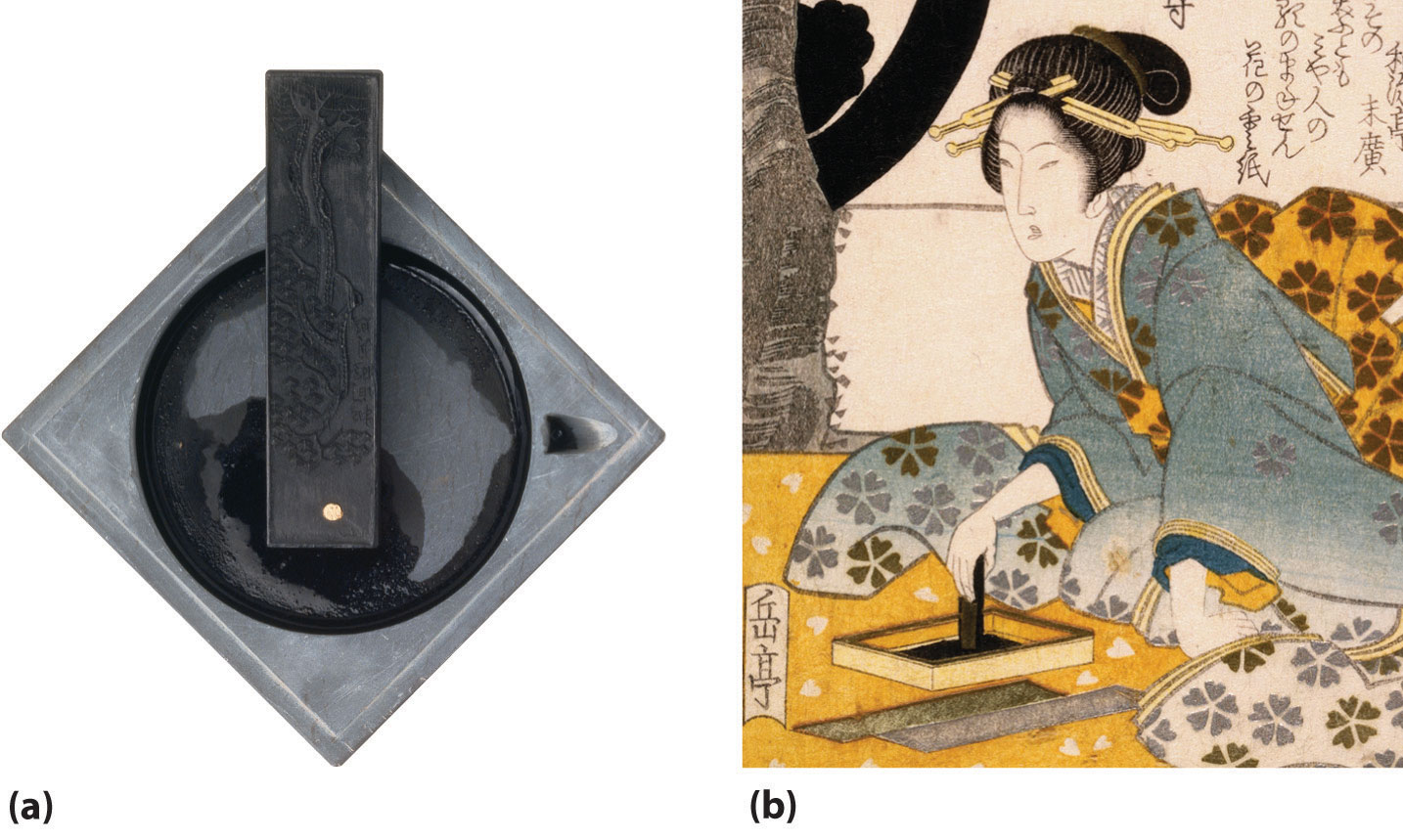
(a) Since ancient times, ink sticks have been the major source of black ink in Asia. Plant oils or resinous woods such as pine are burned, and the resulting soot (lampblack) is collected, mixed with binders such as animal glues and minerals, compressed into a solid stick, and allowed to dry. Liquid ink is made by rubbing the ink stick against the surface of a special stone ink dish with small amounts of water. (b) A 19th-century Japanese painting illustrates how ink is made from an ink stick.
Tin and lead oxides and sulfides are easily reduced to the metal by heating with charcoal, a discovery that must have occurred by accident when prehistoric humans used rocks containing their ores for a cooking fire. However, because tin and copper ores are often found together in nature, their alloy—bronze—was probably discovered before either element, a discovery that led to the Bronze Age. The heaviest element in group 14, lead, is such a soft and malleable metal that the ancient Romans used thin lead foils as writing tablets, as well as lead cookware and lead pipes for plumbing. (Recall that the atomic symbols for tin and lead come from their Latin names: Sn for stannum and Pb for plumbum.)
Although the first glasses were prepared from silica (silicon oxide, SiO2) around 1500 BC, elemental silicon was not prepared until 1824 because of its high affinity for oxygen. Jöns Jakob Berzelius was finally able to obtain amorphous silicon by reducing Na2SiF6 with molten potassium. The crystalline element, which has a shiny blue-gray luster, was not isolated until 30 yr later. The last member of the group 14 elements to be discovered was germanium, which was found in 1886 in a newly discovered silver-colored ore by the German chemist Clemens Winkler, who named the element in honor of his native country.
Preparation and General Properties of the Group 14 Elements
The natural abundance of the group 14 elements varies tremendously. Elemental carbon, for example, ranks only 17th on the list of constituents of Earth’s crust. Pure graphite is obtained by reacting coke, an amorphous form of carbon used as a reductant in the production of steel, with silica to give silicon carbide (SiC). This is then thermally decomposed at very high temperatures (2700°C) to give graphite:
Equation 22.15
Equation 22.16
One allotrope of carbon, diamond, is metastable under normal conditions, with a of 2.9 kJ/mol versus graphite. At pressures greater than 50,000 atm, however, the diamond structure is favored and is the most stable form of carbon. Because the structure of diamond is more compact than that of graphite, its density is significantly higher (3.51 g/cm3 versus 2.2 g/cm3). Because of its high thermal conductivity, diamond powder is used to transfer heat in electronic devices.
The most common sources of diamonds on Earth are ancient volcanic pipes that contain a rock called kimberlite, a lava that solidified rapidly from deep inside the Earth. Most kimberlite formations, however, are much newer than the diamonds they contain. In fact, the relative amounts of different carbon isotopes in diamond show that diamond is a chemical and geological “fossil” older than our solar system, which means that diamonds on Earth predate the existence of our sun. Thus diamonds were most likely created deep inside Earth from primordial grains of graphite present when Earth was formed (part (a) in Figure 22.6 "Crystalline Samples of Carbon and Silicon, the Lightest Group 14 Elements"). Gem-quality diamonds can now be produced synthetically and have chemical, optical, and physical characteristics identical to those of the highest-grade natural diamonds.
Figure 22.6 Crystalline Samples of Carbon and Silicon, the Lightest Group 14 Elements
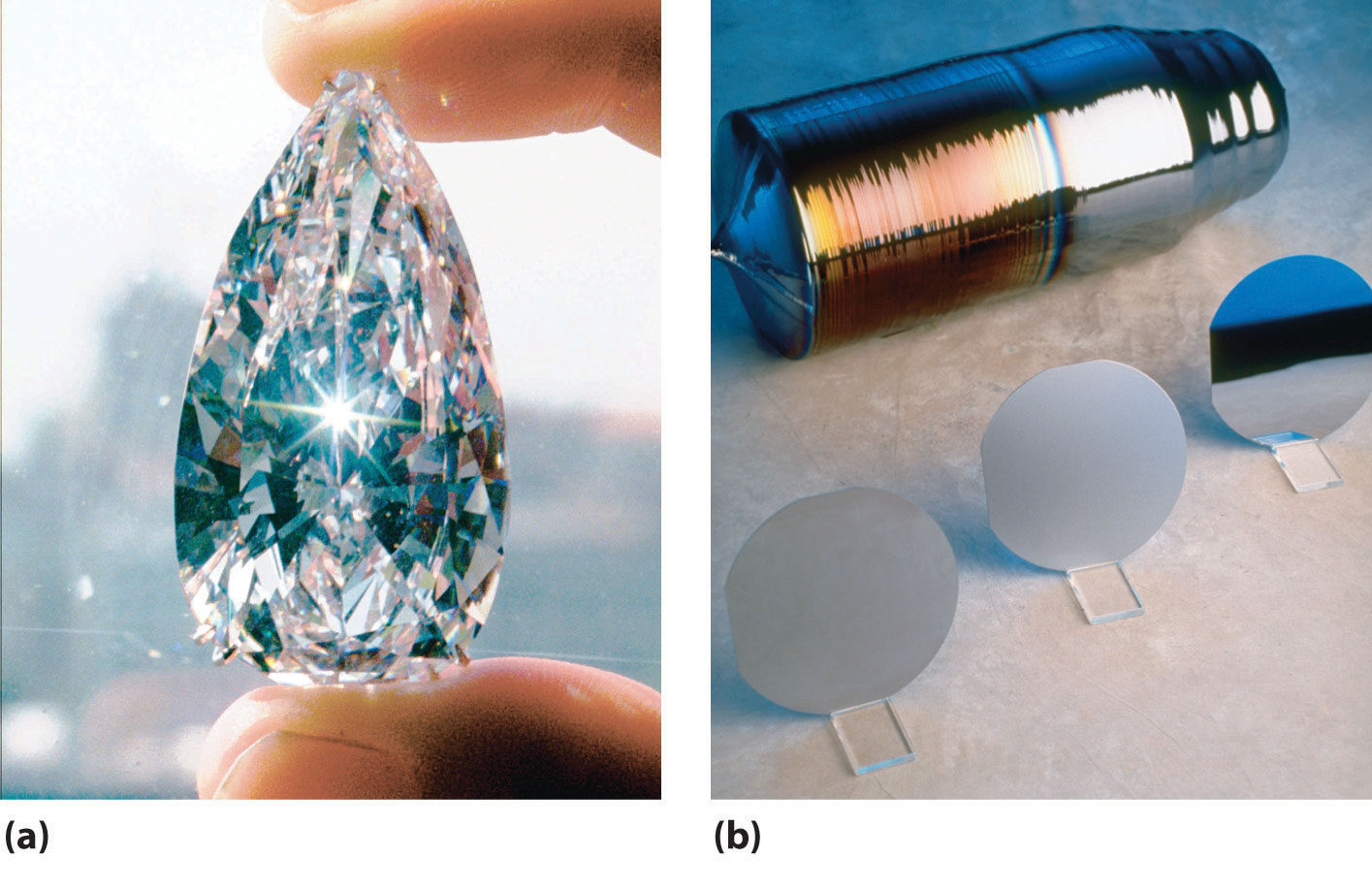
(a) The 78.86-carat Ahmadabad diamond, a historic Indian gem purchased in Gujarat in the 17th century by the French explorer Jean-Baptiste Tavernier and sold in 1995 for $4.3 million, is a rare example of a large single crystal of diamond, the less-stable allotrope of carbon. (b) Large single crystals of highly purified silicon are the basis of the modern electronics industry. They are sliced into very thin wafers that are highly polished and then cut into smaller pieces for use as chips.
Although oxygen is the most abundant element on Earth, the next most abundant is silicon, the next member of group 14. Pure silicon is obtained by reacting impure silicon with Cl2 to give SiCl4, followed by the fractional distillation of the impure SiCl4 and reduction with H2:
Equation 22.17
Several million tons of silicon are annually produced with this method. Amorphous silicon containing residual amounts of hydrogen is used in photovoltaic devices that convert light to electricity, and silicon-based solar cells are used to power pocket calculators, boats, and highway signs, where access to electricity by conventional methods is difficult or expensive. Ultrapure silicon and germanium form the basis of the modern electronics industry (part (b) in Figure 22.6 "Crystalline Samples of Carbon and Silicon, the Lightest Group 14 Elements").
In contrast to silicon, the concentrations of germanium and tin in Earth’s crust are only 1–2 ppm. The concentration of lead, which is the end product of the nuclear decay of many radionuclides, is 13 ppm, making lead by far the most abundant of the heavy group 14 elements. (For more information on radionuclides, see Chapter 20 "Nuclear Chemistry".) No concentrated ores of germanium are known; like indium, germanium is generally recovered from flue dusts obtained by processing the ores of metals such as zinc. Because germanium is essentially transparent to infrared radiation, it is used in optical devices.
Tin and lead are soft metals that are too weak for structural applications, but because tin is flexible, corrosion resistant, and nontoxic, it is used as a coating in food packaging. A “tin can,” for example, is actually a steel can whose interior is coated with a thin layer (1–2 µm) of metallic tin. Tin is also used in superconducting magnets and low-melting-point alloys such as solder and pewter. Pure lead is obtained by heating galena (PbS) in air and reducing the oxide (PbO) to the metal with carbon, followed by electrolytic deposition to increase the purity:
Equation 22.18
Equation 22.19
or
Equation 22.20
By far the single largest use of lead is in lead storage batteries. (For more information on batteries, see Chapter 19 "Electrochemistry".)
As you learned in Chapter 7 "The Periodic Table and Periodic Trends", the group 14 elements all have ns2np2 valence electron configurations. All form compounds in which they formally lose either the two np and the two ns valence electrons or just the two np valence electrons, giving a +4 or +2 oxidation state, respectively. Because covalent bonds decrease in strength with increasing atomic size and the ionization energies for the heavier elements of the group are higher than expected due to a higher Zeff, the relative stability of the +2 oxidation state increases smoothly from carbon to lead.
Note the Pattern
The relative stability of the +2 oxidation state increases, and the tendency to form catenated compounds decreases, from carbon to lead in group 14.
Recall that many carbon compounds contain multiple bonds formed by π overlap of singly occupied 2p orbitals on adjacent atoms. (For more information on atomic orbitals, see Chapter 9 "Molecular Geometry and Covalent Bonding Models".) Compounds of silicon, germanium, tin, and lead with the same stoichiometry as those of carbon, however, tend to have different structures and properties. For example, CO2 is a gas that contains discrete O=C=O molecules, whereas the most common form of SiO2 is the high-melting solid known as quartz, the major component of sand. Instead of discrete SiO2 molecules, quartz contains a three-dimensional network of silicon atoms that is similar to the structure of diamond but with an oxygen atom inserted between each pair of silicon atoms. Thus each silicon atom is linked to four other silicon atoms by bridging oxygen atoms. (For more information on the properties of solids, see Chapter 12 "Solids", Section 12.1 "Crystalline and Amorphous Solids".) The tendency to catenate—to form chains of like atoms—decreases rapidly as we go down group 14 because bond energies for both the E–E and E–H bonds decrease with increasing atomic number (where E is any group 14 element). Consequently, inserting a CH2 group into a linear hydrocarbon such as n-hexane is exergonic (ΔG° = −45 kJ/mol), whereas inserting an SiH2 group into the silicon analogue of n-hexane (Si6H14) actually costs energy (ΔG° ≈ +25 kJ/mol). As a result of this trend, the thermal stability of catenated compounds decreases rapidly from carbon to lead.
In Table 22.2 "Selected Properties of the Group 14 Elements" we see, once again, that there is a large difference between the lightest element (C) and the others in size, ionization energy, and electronegativity. As in group 13, the second and third elements (Si and Ge) are similar, and there is a reversal in the trends for some properties, such as ionization energy, between the fourth and fifth elements (Sn and Pb). As for group 13, these effects can be explained by the presence of filled (n − 1)d and (n − 2)f subshells, whose electrons are relatively poor at screening the outermost electrons from the higher nuclear charge.
Table 22.2 Selected Properties of the Group 14 Elements
| Property | Carbon | Silicon | Germanium | Tin | Lead |
|---|---|---|---|---|---|
| atomic symbol | C | Si | Ge | Sn | Pb |
| atomic number | 6 | 14 | 32 | 50 | 82 |
| atomic mass (amu) | 12.01 | 28.09 | 72.64 | 118.71 | 207.2 |
| valence electron configuration* | 2s22p2 | 3s23p2 | 4s24p2 | 5s25p2 | 6s26p2 |
| melting point/boiling point (°C) | 4489 (at 10.3 MPa)/3825 | 1414/3265 | 939/2833 | 232/2602 | 327/1749 |
| density (g/cm3) at 25°C | 2.2 (graphite), 3.51 (diamond) | 2.33 | 5.32 | 7.27(white) | 11.30 |
| atomic radius (pm) | 77 (diamond) | 111 | 125 | 145 | 154 |
| first ionization energy (kJ/mol) | 1087 | 787 | 762 | 709 | 716 |
| most common oxidation state | +4 | +4 | +4 | +4 | +4 |
| ionic radius (pm)† | ≈29 | ≈40 | 53 | 69 | 77.5 |
| electron affinity (kJ/mol) | −122 | −134 | −119 | −107 | −35 |
| electronegativity | 2.6 | 1.9 | 2.0 | 2.0 | 1.8 |
| standard reduction potential (E°, V) (for EO2 → E in acidic solution) | 0.21 | −0.86 | −0.18 | −0.12 | 0.79 |
| product of reaction with O2 | CO2, CO | SiO2 | GeO2 | SnO2 | PbO |
| type of oxide | acidic (CO2) | acidic neutral (CO) | amphoteric | amphoteric | amphoteric |
| product of reaction with N2 | none | Si3N4 | none | Sn3N4 | none |
| product of reaction with X2‡ | CX4 | SiX4 | GeX4 | SnX4 | PbX2 |
| product of reaction with H2 | CH4 | none | none | none | none |
| *The configuration shown does not include filled d and f subshells. | |||||
| †The values cited are for six-coordinate +4 ions in the most common oxidation state, except for C4+ and Si4+, for which values for the four-coordinate ion are estimated. | |||||
| ‡X is Cl, Br, or I. Reaction with F2 gives the tetrafluorides (EF4) for all group 14 elements, where E represents any group 14 element. | |||||
Note the Pattern
The group 14 elements follow the same pattern as the group 13 elements in their periodic properties.
Reactions and Compounds of Carbon
Carbon is the building block of all organic compounds, including biomolecules, fuels, pharmaceuticals, and plastics, whereas inorganic compounds of carbon include metal carbonates, which are found in substances as diverse as fertilizers and antacid tablets, halides, oxides, carbides, and carboranes. Like boron in group 13, the chemistry of carbon differs sufficiently from that of its heavier congeners to merit a separate discussion.
The structures of the allotropes of carbon—diamond, graphite, fullerenes, and nanotubes—are distinct, but they all contain simple electron-pair bonds (Figure 7.18 "Four Allotropes of Carbon"). Although it was originally believed that fullerenes were a new form of carbon that could be prepared only in the laboratory, fullerenes have been found in certain types of meteorites. Another possible allotrope of carbon has also been detected in impact fragments of a carbon-rich meteorite; it appears to consist of long chains of carbon atoms linked by alternating single and triple bonds, (–C≡C–C≡C–)n. Carbon nanotubes (“buckytubes”) are being studied as potential building blocks for ultramicroscale detectors and molecular computers and as tethers for space stations. They are currently used in electronic devices, such as the electrically conducting tips of miniature electron guns for flat-panel displays in portable computers.
Although all the carbon tetrahalides (CX4) are known, they are generally not obtained by the direct reaction of carbon with the elemental halogens (X2) but by indirect methods such as the following reaction, where X is Cl or Br:
Equation 22.21
CH4(g) + 4X2(g) → CX4(l,s) + 4HX(g)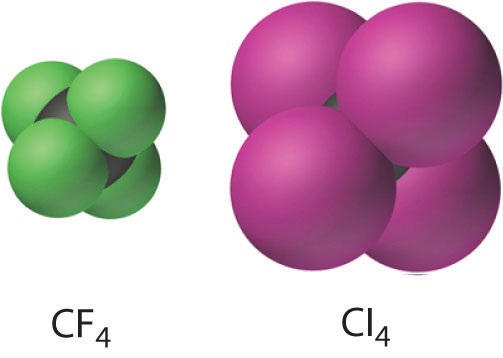
The carbon tetrahalides all have the tetrahedral geometry predicted by the valence-shell electron-pair repulsion (VSEPR) model, as shown for CCl4 and CI4. Their stability decreases rapidly as the halogen increases in size because of poor orbital overlap and increased crowding. Because the C–F bond is about 25% stronger than a C–H bond, fluorocarbons are thermally and chemically more stable than the corresponding hydrocarbons, while having a similar hydrophobic character. A polymer of tetrafluoroethylene (F2C=CF2), analogous to polyethylene, is the nonstick Teflon lining found on many cooking pans, and similar compounds are used to make fabrics stain resistant (such as Scotch-Gard) or waterproof but breathable (such as Gore-Tex).
Note the Pattern
The stability of the carbon tetrahalides decreases with increasing size of the halogen due to increasingly poor orbital overlap and crowding.
Carbon reacts with oxygen to form either CO or CO2, depending on the stoichiometry. Carbon monoxide is a colorless, odorless, and poisonous gas that reacts with the iron in hemoglobin to form an Fe–CO unit, which prevents hemoglobin from binding, transporting, and releasing oxygen in the blood (see Figure 23.26 "Binding of O" for myoglobin). In the laboratory, carbon monoxide can be prepared on a small scale by dehydrating formic acid with concentrated sulfuric acid:
Equation 22.22
Carbon monoxide also reacts with the halogens to form the oxohalides (COX2). Probably the best known of these is phosgene (Cl2C=O), which is highly poisonous and was used as a chemical weapon during World War I:
Equation 22.23
Despite its toxicity, phosgene is an important industrial chemical that is prepared on a large scale, primarily in the manufacture of polyurethanes.
Carbon dioxide can be prepared on a small scale by reacting almost any metal carbonate or bicarbonate salt with a strong acid. As is typical of a nonmetal oxide, CO2 reacts with water to form acidic solutions containing carbonic acid (H2CO3). In contrast to its reactions with oxygen, reacting carbon with sulfur at high temperatures produces only carbon disulfide (CS2):
Equation 22.24
The selenium analogue CSe2 is also known. Both have the linear structure predicted by the VSEPR model, and both are vile smelling (and in the case of CSe2, highly toxic), volatile liquids. The sulfur and selenium analogues of carbon monoxide, CS and CSe, are unstable because the C≡Y bonds (Y is S or Se) are much weaker than the C≡O bond due to poorer π orbital overlap.
Note the Pattern
Pi bonds between carbon and the heavier chalcogenides are weak due to poor orbital overlap.
Binary compounds of carbon with less electronegative elements are called carbides. The chemical and physical properties of carbides depend strongly on the identity of the second element, resulting in three general classes: ionic carbides, interstitial carbides, and covalent carbides. The reaction of carbon at high temperatures with electropositive metals such as those of groups 1 and 2 and aluminum produces ionic carbides, which contain discrete metal cations and carbon anions. The identity of the anions depends on the size of the second element. For example, smaller elements such as beryllium and aluminum give methides such as Be2C and Al4C3, which formally contain the C4− ion derived from methane (CH4) by losing all four H atoms as protons. In contrast, larger metals such as sodium and calcium give carbides with stoichiometries of Na2C2 and CaC2. Because these carbides contain the C4− ion, which is derived from acetylene (HC≡CH) by losing both H atoms as protons, they are more properly called acetylides. As discussed in Chapter 21 "Periodic Trends and the ", Section 21.4 "The Alkaline Earth Metals (Group 2)", reacting ionic carbides with dilute aqueous acid results in protonation of the anions to give the parent hydrocarbons: CH4 or C2H2. For many years, miners’ lamps used the reaction of calcium carbide with water to produce a steady supply of acetylene, which was ignited to provide a portable lantern.

19th-century miner’s lamp. The lamp uses burning acetylene, produced by the slow reaction of calcium carbide with water, to provide light.
The reaction of carbon with most transition metals at high temperatures produces interstitial carbides. Due to the less electropositive nature of the transition metals, these carbides contain covalent metal–carbon interactions, which result in different properties: most interstitial carbides are good conductors of electricity, have high melting points, and are among the hardest substances known. Interstitial carbides exhibit a variety of nominal compositions, and they are often nonstoichiometric compounds whose carbon content can vary over a wide range. Among the most important are tungsten carbide (WC), which is used industrially in high-speed cutting tools, and cementite (Fe3C), which is a major component of steel.
Elements with an electronegativity similar to that of carbon form covalent carbides, such as silicon carbide (SiC; Equation 22.15) and boron carbide (B4C). These substances are extremely hard, have high melting points, and are chemically inert. For example, silicon carbide is highly resistant to chemical attack at temperatures as high as 1600°C. Because it also maintains its strength at high temperatures, silicon carbide is used in heating elements for electric furnaces and in variable-temperature resistors.
Note the Pattern
Carbides formed from group 1 and 2 elements are ionic. Transition metals form interstitial carbides with covalent metal–carbon interactions, and covalent carbides are chemically inert.
Example 3
For each reaction, explain why the given product forms.
- CO(g) + Cl2(g) → Cl2C=O(g)
- CO(g) + BF3(g) → F3B:C≡O(g)
- Sr(s) + 2C(s) SrC2(s)
Given: balanced chemical equations
Asked for: why the given products form
Strategy:
Classify the type of reaction. Using periodic trends in atomic properties, thermodynamics, and kinetics, explain why the observed reaction products form.
Solution:
- Because the carbon in CO is in an intermediate oxidation state (+2), CO can be either a reductant or an oxidant; it is also a Lewis base. The other reactant (Cl2) is an oxidant, so we expect a redox reaction to occur in which the carbon of CO is further oxidized. Because Cl2 is a two-electron oxidant and the carbon atom of CO can be oxidized by two electrons to the +4 oxidation state, the product is phosgene (Cl2C=O).
- Unlike Cl2, BF3 is not a good oxidant, even though it contains boron in its highest oxidation state (+3). Nor can BF3 behave like a reductant. Like any other species with only six valence electrons, however, it is certainly a Lewis acid. Hence an acid–base reaction is the most likely alternative, especially because we know that CO can use the lone pair of electrons on carbon to act as a Lewis base. The most probable reaction is therefore the formation of a Lewis acid–base adduct.
- Typically, both reactants behave like reductants. Unless one of them can also behave like an oxidant, no reaction will occur. We know that Sr is an active metal because it lies far to the left in the periodic table and that it is more electropositive than carbon. Carbon is a nonmetal with a significantly higher electronegativity; it is therefore more likely to accept electrons in a redox reaction. We conclude, therefore, that Sr will be oxidized, and C will be reduced. Carbon forms ionic carbides with active metals, so the reaction will produce a species formally containing either C4− or C22−. Those that contain C4− usually involve small, highly charged metal ions, so Sr2+ will produce the acetylide (SrC2) instead.
Exercise
Predict the products of the reactions and write a balanced chemical equation for each reaction.
- C(s) + excess O2(g)
- C(s) + H2O(l) →
- NaHCO3(s) + H2SO4(aq) →
Answer:
- C(s) + excess O2(g) CO2(g)
- C(s) + H2O(l) → no reaction
- NaHCO3(s) + H2SO4(aq) → CO2(g) + NaHSO4(aq) + H2O(l)
Reactions and Compounds of the Heavier Group 14 Elements
Although silicon, germanium, tin, and lead in their +4 oxidation states often form binary compounds with the same stoichiometry as carbon, the structures and properties of these compounds are usually significantly different from those of the carbon analogues. Silicon and germanium are both semiconductors with structures analogous to diamond. Tin has two common allotropes: white (β) tin has a metallic lattice and metallic properties, whereas gray (α) tin has a diamond-like structure and is a semiconductor. The metallic β form is stable above 13.2°C, and the nonmetallic α form is stable below 13.2°C. Lead is the only group 14 element that is metallic in both structure and properties under all conditions.
Based on its position in the periodic table, we expect silicon to be amphoteric. In fact, it dissolves in strong aqueous base to produce hydrogen gas and solutions of silicates, but the only aqueous acid that it reacts with is hydrofluoric acid, presumably due to the formation of the stable SiF62− ion. Germanium is more metallic in its behavior than silicon. For example, it dissolves in hot oxidizing acids, such as HNO3 and H2SO4, but in the absence of an oxidant, it does not dissolve in aqueous base. Although tin has an even more metallic character than germanium, lead is the only element in the group that behaves purely as a metal. Acids do not readily attack it because the solid acquires a thin protective outer layer of a Pb2+ salt, such as PbSO4.
All group 14 dichlorides are known, and their stability increases dramatically as the atomic number of the central atom increases. Thus CCl2 is dichlorocarbene, a highly reactive, short-lived intermediate that can be made in solution but cannot be isolated in pure form using standard techniques; SiCl2 can be isolated at very low temperatures, but it decomposes rapidly above −150°C, and GeCl2 is relatively stable at temperatures below 20°C. In contrast, SnCl2 is a polymeric solid that is indefinitely stable at room temperature, whereas PbCl2 is an insoluble crystalline solid with a structure similar to that of SnCl2.
Note the Pattern
The stability of the group 14 dichlorides increases dramatically from carbon to lead.
Although the first four elements of group 14 form tetrahalides (MX4) with all the halogens, only fluorine is able to oxidize lead to the +4 oxidation state, giving PbF4. The tetrahalides of silicon and germanium react rapidly with water to give amphoteric oxides (where M is Si or Ge):
Equation 22.25
MX4(s,l) + 2H2O(l) → MO2(s) + 4HX(aq)In contrast, the tetrahalides of tin and lead react with water to give hydrated metal ions.
Because of the stability of its +2 oxidation state, lead reacts with oxygen or sulfur to form PbO or PbS, respectively, whereas heating the other group 14 elements with excess O2 or S8 gives the corresponding dioxides or disulfides, respectively. The dioxides of the group 14 elements become increasingly basic as we go down the group.
Note the Pattern
The dioxides of the group 14 elements become increasingly basic down the group.
Because the Si–O bond is even stronger than the C–O bond (∼452 kJ/mol versus ∼358 kJ/mol), silicon has a strong affinity for oxygen. The relative strengths of the C–O and Si–O bonds contradict the generalization that bond strengths decrease as the bonded atoms become larger. This is because we have thus far assumed that a formal single bond between two atoms can always be described in terms of a single pair of shared electrons. In the case of Si–O bonds, however, the presence of relatively low-energy, empty d orbitals on Si and nonbonding electron pairs in the p or spn hybrid orbitals of O results in a partial π bond (Figure 22.7 "Pi Bonding between Silicon and Oxygen"). Due to its partial π double bond character, the Si–O bond is significantly stronger and shorter than would otherwise be expected. A similar interaction with oxygen is also an important feature of the chemistry of the elements that follow silicon in the third period (P, S, and Cl). Because the Si–O bond is unusually strong, silicon–oxygen compounds dominate the chemistry of silicon.
Figure 22.7 Pi Bonding between Silicon and Oxygen
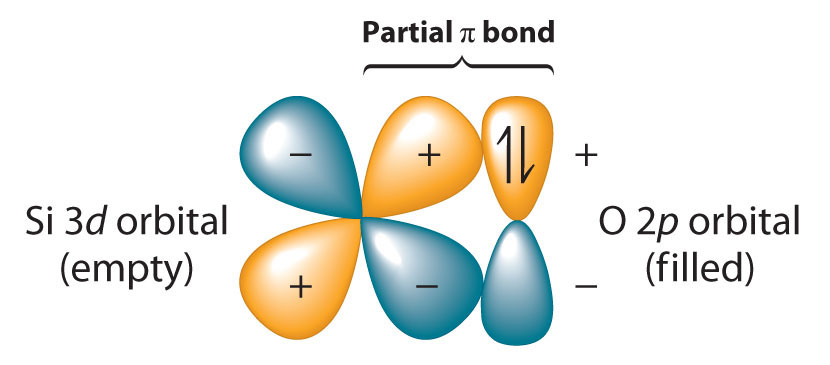
Silicon has relatively low-energy, empty 3d orbitals that can interact with filled 2p hybrid orbitals on oxygen. This interaction results in a partial π bond in which both electrons are supplied by oxygen, giving the Si–O bond partial double bond character and making it significantly stronger (and shorter) than expected for a single bond.
Note the Pattern
Because silicon–oxygen bonds are unusually strong, silicon–oxygen compounds dominate the chemistry of silicon.
Compounds with anions that contain only silicon and oxygen are called silicates, whose basic building block is the SiO44− unit:
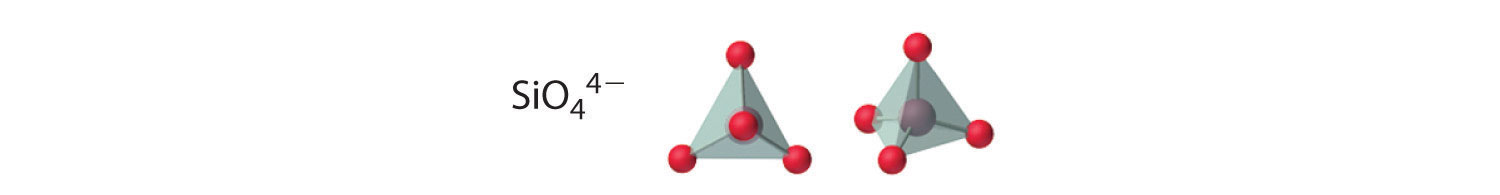
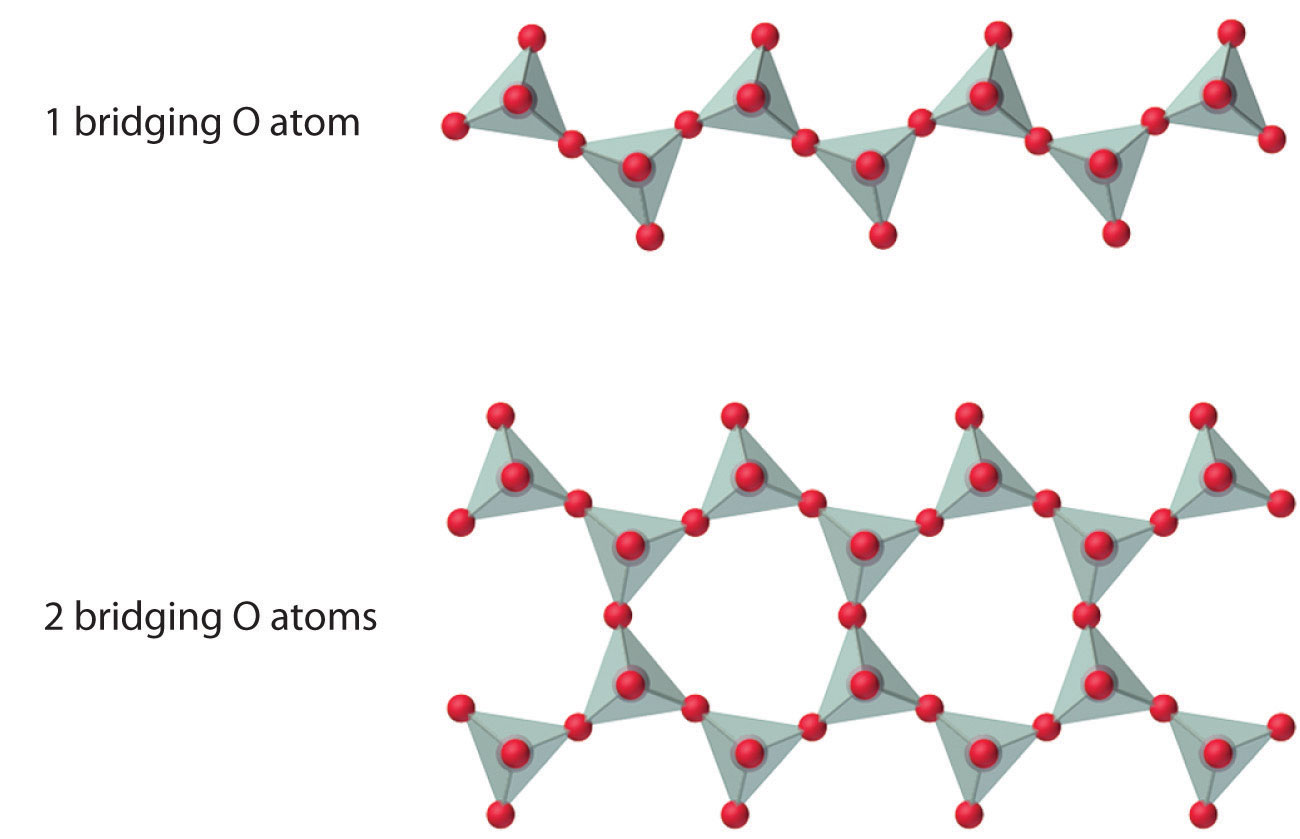
The number of oxygen atoms shared between silicon atoms and the way in which the units are linked vary considerably in different silicates. Converting one of the oxygen atoms from terminal to bridging generates chains of silicates, while converting two oxygen atoms from terminal to bridging generates double chains. In contrast, converting three or four oxygens to bridging generates a variety of complex layered and three-dimensional structures, respectively.

Opal gemstones.
The silicates include many glasses as well as the gemstone known as opal, which typically contains 10%–15% water. In a large and important class of materials called aluminosilicates, some of the Si atoms are replaced by Al atoms to give aluminosilicates such as zeolites, whose three-dimensional framework structures have large cavities connected by smaller tunnels (Figure 22.8 "Zeolites Are Aluminosilicates with Large Cavities Connected by Channels"). Because the cations in zeolites are readily exchanged, zeolites are used in laundry detergents as water-softening agents: the more loosely bound Na+ ions inside the zeolite cavities are displaced by the more highly charged Mg2+ and Ca2+ ions present in hard water, which bind more tightly. Zeolites are also used as catalysts and for water purification.
Figure 22.8 Zeolites Are Aluminosilicates with Large Cavities Connected by Channels
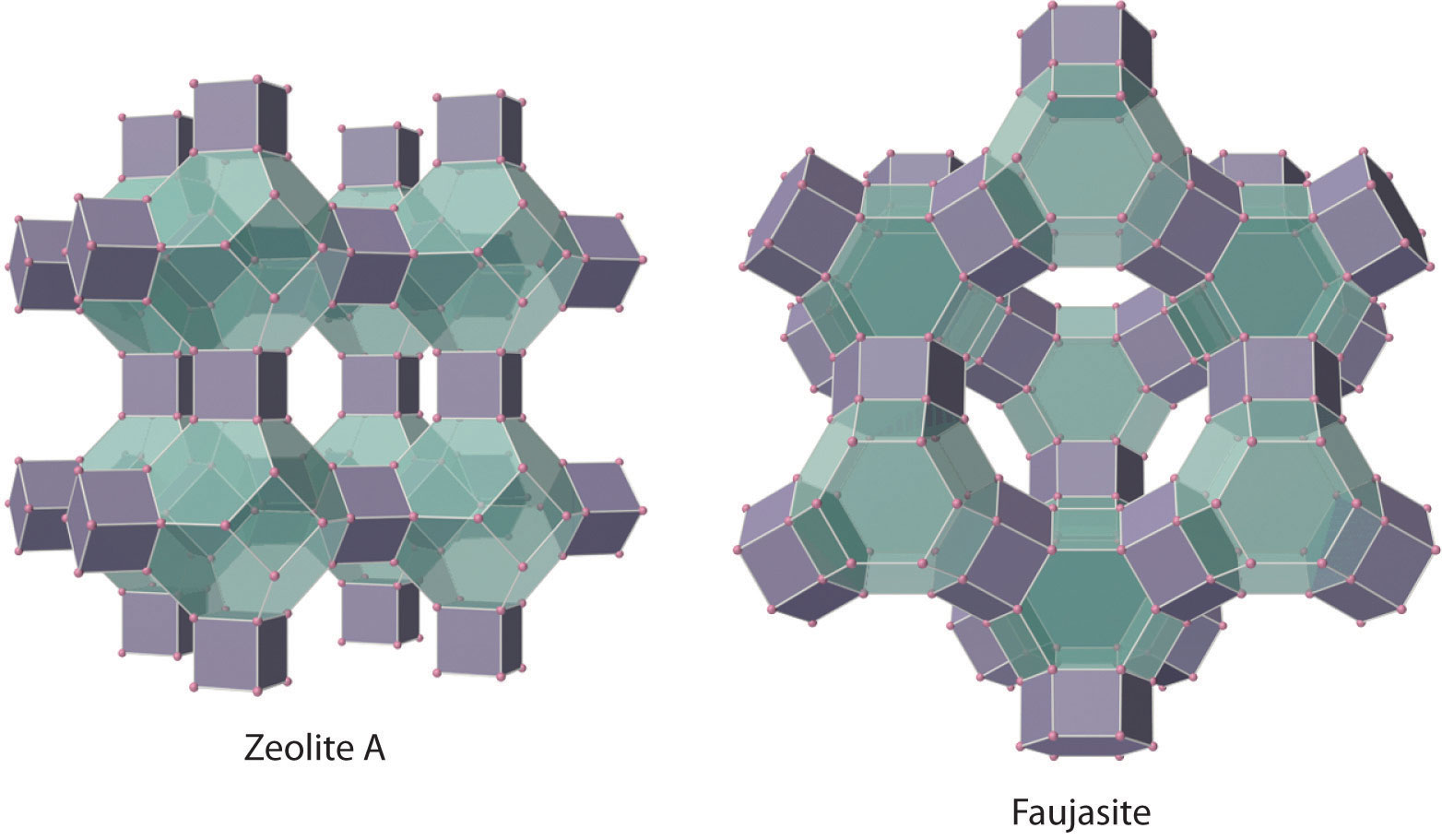
The cavities normally contain hydrated cations that are loosely bound to the oxygen atoms of the negatively charged framework by electrostatic interactions. The sizes and arrangements of the channels and cavities differ in different types of zeolites. For example, in zeolite A the aluminosilicate cages are arranged in a cubic fashion, and the channels connecting the cavities intersect at right angles. In contrast, the cavities in faujasite are much larger, and the channels intersect at 120° angles. In these idealized models, the oxygen atoms that connect each pair of silicon atoms have been omitted.
Silicon and germanium react with nitrogen at high temperature to form nitrides (M3N4):
Equation 22.26
3Si(l) + 2N2(g) → Si3N4(s)Silicon nitride has properties that make it suitable for high-temperature engineering applications: it is strong, very hard, and chemically inert, and it retains these properties to temperatures of about 1000°C.
Because of the diagonal relationship between boron and silicon, metal silicides and metal borides exhibit many similarities. Although metal silicides have structures that are as complex as those of the metal borides and carbides, few silicides are structurally similar to the corresponding borides due to the significantly larger size of Si (atomic radius 111 pm versus 87 pm for B). Silicides of active metals, such as Mg2Si, are ionic compounds that contain the Si4− ion. They react with aqueous acid to form silicon hydrides such as SiH4:
Equation 22.27
Mg2Si(s) + 4H+(aq) → 2Mg2+(aq) + SiH4(g)Unlike carbon, catenated silicon hydrides become thermodynamically less stable as the chain lengthens. Thus straight-chain and branched silanes (analogous to alkanes) are known up to only n = 10; the germanium analogues (germanes) are known up to n = 9. In contrast, the only known hydride of tin is SnH4, and it slowly decomposes to elemental Sn and H2 at room temperature. The simplest lead hydride (PbH4) is so unstable that chemists are not even certain it exists. Because E=E and E≡E bonds become weaker with increasing atomic number (where E is any group 14 element), simple silicon, germanium, and tin analogues of alkenes, alkynes, and aromatic hydrocarbons are either unstable (Si=Si and Ge=Ge) or unknown. Silicon-based life-forms are therefore likely to be found only in science fiction.
Note the Pattern
The stability of group 14 hydrides decreases down the group, and E=E and E≡E bonds become weaker.
The only important organic derivatives of lead are compounds such as tetraethyllead [(CH3CH2)4Pb]. Because the Pb–C bond is weak, these compounds decompose at relatively low temperatures to produce alkyl radicals (R·), which can be used to control the rate of combustion reactions. For 60 yr, hundreds of thousands of tons of lead were burned annually in automobile engines, producing a mist of lead oxide particles along the highways that constituted a potentially serious public health problem. (Example 6 in Section 22.3 "The Elements of Group 15 (The Pnicogens)" examines this problem.) The use of catalytic converters reduced the amount of carbon monoxide, nitrogen oxides, and hydrocarbons released into the atmosphere through automobile exhausts, but it did nothing to decrease lead emissions. Because lead poisons catalytic converters, however, its use as a gasoline additive has been banned in most of the world.
Compounds that contain Si–C and Si–O bonds are stable and important. High-molecular-mass polymers called silicones contain an (Si–O–)n backbone with organic groups attached to Si (Figure 22.9 "Silicones Are Polymers with Long Chains of Alternating Silicon and Oxygen Atoms"). The properties of silicones are determined by the chain length, the type of organic group, and the extent of cross-linking between the chains. Without cross-linking, silicones are waxes or oils, but cross-linking can produce flexible materials used in sealants, gaskets, car polishes, lubricants, and even elastic materials, such as the plastic substance known as Silly Putty.
Figure 22.9 Silicones Are Polymers with Long Chains of Alternating Silicon and Oxygen Atoms
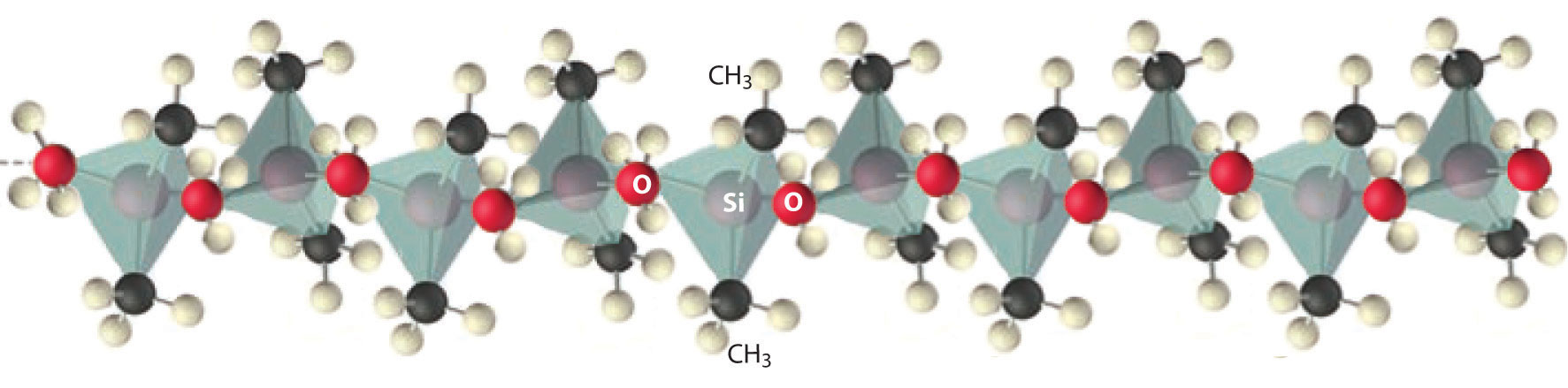
The structure of a linear silicone polymer is similar to that of quartz, but two of the oxygen atoms attached to each silicon atom are replaced by the carbon atoms of organic groups, such as the methyl groups (–CH3) shown here. The terminal silicon atoms are bonded to three methyl groups. Silicones can be oily, waxy, flexible, or elastic, depending on the chain length, the extent of cross-linking between the chains, and the type of organic group.

A child playing with Silly Putty, a silicone polymer with unusual mechanical properties. Gentle pressure causes Silly Putty to flow or stretch, but it cannot be flattened when hit with a hammer.
Example 4
For each reaction, explain why the given products form.
- Pb(s) + Cl2(g) → PbCl2(s)
- Mg2Si(s) + 4H2O(l) → SiH4(g) + 2Mg(OH)2(s)
- GeO2(s) + 4OH−(aq) → GeO44−(aq) + 2H2O(l)
Given: balanced chemical equations
Asked for: why the given products form
Strategy:
Classify the type of reaction. Using periodic trends in atomic properties, thermodynamics, and kinetics, explain why the observed reaction products form.
Solution:
- Lead is a metal, and chlorine is a nonmetal that is a strong oxidant. Thus we can expect a redox reaction to occur in which the metal acts as a reductant. Although lead can form compounds in the +2 and +4 oxidation states, Pb4+ is a potent oxidant (the inert-pair effect). Because lead prefers the +2 oxidation state and chlorine is a weaker oxidant than fluorine, we expect PbCl2 to be the product.
- This is the reaction of water with a metal silicide, which formally contains the Si4− ion. Water can act as either an acid or a base. Because the other compound is a base, we expect an acid–base reaction to occur in which water acts as an acid. Because Mg2Si contains Si in its lowest possible oxidation state, however, an oxidation–reduction reaction is also a possibility. But water is a relatively weak oxidant, so an acid–base reaction is more likely. The acid (H2O) transfers a proton to the base (Si4−), which can accept four protons to form SiH4. Proton transfer from water produces the OH− ion, which will combine with Mg2+ to give magnesium hydroxide.
- We expect germanium dioxide (GeO2) to be amphoteric because of the position of germanium in the periodic table. It should dissolve in strong aqueous base to give an anionic species analogous to silicate.
Exercise
Predict the products of the reactions and write a balanced chemical equation for each reaction.
- PbO2(s)
- GeCl4(s) + H2O(l) →
- Sn(s) + HCl(aq) →
Answer:
- GeCl4(s) + 2H2O(l) → GeO2(s) + 4HCl(aq)
- Sn(s) + 2HCl(aq) → Sn2+(aq) + H2(g) + 2Cl−(aq)
Summary
The group 14 elements show the greatest range of chemical behavior of any group in the periodic table. Because the covalent bond strength decreases with increasing atomic size and greater-than-expected ionization energies due to an increase in Zeff, the stability of the +2 oxidation state increases from carbon to lead. The tendency to form multiple bonds and to catenate decreases as the atomic number increases. The stability of the carbon tetrahalides decreases as the halogen increases in size because of poor orbital overlap and steric crowding. Carbon forms three kinds of carbides with less electronegative elements: ionic carbides, which contain metal cations and C4− (methide) or C22− (acetylide) anions; interstitial carbides, which are characterized by covalent metal–carbon interactions and are among the hardest substances known; and covalent carbides, which have three-dimensional covalent network structures that make them extremely hard, high melting, and chemically inert. Consistent with periodic trends, metallic behavior increases down the group. Silicon has a tremendous affinity for oxygen because of partial Si–O π bonding. Dioxides of the group 14 elements become increasingly basic down the group and their metallic character increases. Silicates contain anions that consist of only silicon and oxygen. Aluminosilicates are formed by replacing some of the Si atoms in silicates by Al atoms; aluminosilicates with three-dimensional framework structures are called zeolites. Nitrides formed by reacting silicon or germanium with nitrogen are strong, hard, and chemically inert. The hydrides become thermodynamically less stable down the group. Moreover, as atomic size increases, multiple bonds between or to the group 14 elements become weaker. Silicones, which contain an Si–O backbone and Si–C bonds, are high-molecular-mass polymers whose properties depend on their compositions.
Key Takeaway
- The group 14 elements show the greatest diversity in chemical behavior of any group; covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected, increasing from C to Pb.
Conceptual Problems
-
Why is the preferred oxidation state of lead +2 rather than +4? What do you expect the preferred oxidation state of silicon to be based on its position in the periodic table?
-
Carbon uses pπ–pπ overlap to form compounds with multiple bonds, but silicon does not. Why? How does this same phenomenon explain why the heavier elements in group 14 do not form catenated compounds?
-
Diamond is both an electrical insulator and an excellent thermal conductor. Explain this property in terms of its bonding.
-
The lighter chalcogens (group 16) form π bonds with carbon. Does the strength of these π bonds increase or decrease with increasing atomic number of the chalcogen? Why?
-
The heavier group 14 elements can form complexes that contain expanded coordination spheres. How does this affect their reactivity compared with the reactivity of carbon? Is this a thermodynamic effect or a kinetic effect? Explain your answer.
-
Refer to Table 22.2 "Selected Properties of the Group 14 Elements" for the values of the electron affinities of the group 14 elements. Explain any discrepancies between these actual values and the expected values based on usual periodic trends.
-
Except for carbon, the elements of group 14 can form five or six electron-pair bonds. What hybrid orbitals are used to allow this expanded coordination? Why does carbon not form more than four electron-pair bonds?
-
Which of the group 14 elements is least stable in the +4 oxidation state? Why?
Structure and Reactivity
-
Predict the products of each reaction and balance each chemical equation.
- CaC2(s) + HCl(g) →
- Pb(s) + Br2(l)
- (CH3)3N(l) + H2O2(aq) →
- Pb(N3)2(s)
-
Write a balanced chemical equation to indicate how you would prepare each compound.
- SiF62− from its elements and other common compounds
- SiO2 from SiCl4
- GeS2 from its elements
- Si(CH3)4 from Si
-
Write a balanced chemical equation to indicate how you would prepare each compound.
- CO2 from CuO
- methane from Be2C
- Si(OH)4 from Si
- Si3N4 from Si
Answers
-
- CaC2(s) + 2HCl(g) → CaCl2(s) + C2H2(g)
- Pb(s) + Br2(l) PbBr2(s)
- (CH3)3N(l) + H2O2(aq) → (CH3)3N–O(l) + H2O(l)
- Pb(N3)2(s) Pb(s) + 3N2(g)
-
-
- CuO(s) + CO(s) Cu(s) + CO2(g)
- Be2C(s) + 4HCl(aq) → 2BeCl2(aq) + CH4(g)
- Si(s) + 2Cl2(g) → SiCl4(l); SiCl4(l) + 4H2O(l) → Si(OH)4(s) + 4HCl(aq)
- 3Si(s) + 2N2(g) Si3N4(s)




