This is “End-of-Chapter Material”, section 16.7 from the book Principles of General Chemistry (v. 1.0M). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
16.7 End-of-Chapter Material
Application Problems
-
The analytical concentration of lactic acid in blood is generally less than 1.2 × 10−3 M, corresponding to the sum of [lactate] and [lactic acid]. During strenuous exercise, however, oxygen in the muscle tissue is depleted, and overproduction of lactic acid occurs. This leads to a condition known as lactic acidosis, which is characterized by elevated blood lactic acid levels (approximately 5.0 × 10−3 M). The pKa of lactic acid is 3.86.
- What is the actual lactic acid concentration under normal physiological conditions?
- What is the actual lactic acid concentration during lactic acidosis?
-
When the internal temperature of a human reaches 105°F, immediate steps must be taken to prevent the person from having convulsions. At this temperature, Kw is approximately 2.94 × 10−14.
- Calculate the pKw and the pH and pOH of a neutral solution at 105°F.
- Is the pH greater than or less than that calculated in Exercise 1 for a neutral solution at a normal body temperature of 98.6°F?
-
♦ The compound diphenhydramine (DPH) is the active ingredient in a number of over-the-counter antihistamine medications used to treat the runny nose and watery eyes associated with hay fever and other allergies. DPH is a derivative of trimethylamine (one methyl group is replaced by a more complex organic “arm” containing two phenyl rings):
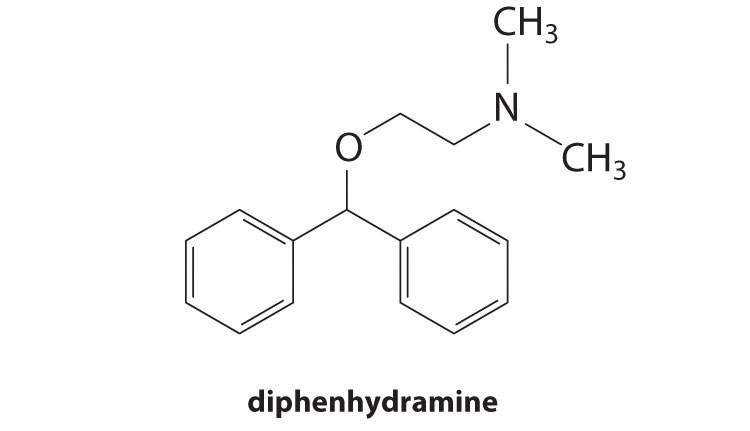
The compound is sold as the water-soluble hydrochloride salt (DPH+Cl−). A tablet of diphenhydramine hydrochloride contains 25.0 mg of the active ingredient. Calculate the pH of the solution if two tablets are dissolved in 100 mL of water. The pKb of diphenhydramine is 5.47, and the formula mass of diphenhydramine hydrochloride is 291.81 amu.
-
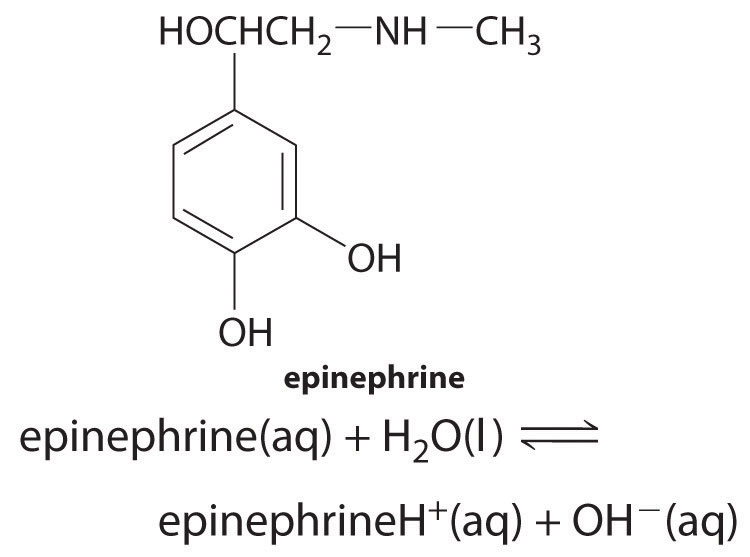
♦ Epinephrine, a secondary amine, is used to counter allergic reactions as well as to bring patients out of anesthesia and cardiac arrest. The pKb of epinephrine is 4.31. What is the percent ionization in a 0.280 M solution? What is the percent ionization after enough solid epinephrine hydrochloride is added to make the final epinephrineH+ concentration 0.982 M? What is the final pH of the solution?
-
♦ Fluoroacetic acid is a poison that has been used by ranchers in the western United States. The ranchers place the poison in the carcasses of dead animals to kill coyotes that feed on them; unfortunately, however, eagles and hawks are also killed in the process. How many milliliters of 0.0953 M Ca(OH)2 are needed to completely neutralize 50.0 mL of 0.262 M fluoroacetic acid solution (pKa = 2.59)? What is the initial pH of the solution? What is the pH of the solution at the equivalence point?
-
Accidental ingestion of aspirin (acetylsalicylic acid) is probably the most common cause of childhood poisoning. Initially, salicylates stimulate the portion of the brain that controls breathing, resulting in hyperventilation (excessively intense breathing that lowers the in the lungs). Subsequently, a potentially serious rebound effect occurs, as the salicylates are converted to a weak acid, salicylic acid, in the body. Starting with the normal values of = 40 mmHg, pH = 7.40, and [HCO3−] = 24 nM, show what happens during the initial phase of respiratory stimulation and the subsequent phase of acid production. Why is the rebound effect dangerous?
-
Emphysema is a disease that reduces the efficiency of breathing. As a result, less CO2 is exchanged with the atmosphere. What effect will this have on blood pH, and [HCO3−]?
Problems marked with a ♦ involve multiple concepts.
Answer
-
-
-
-
-
68.7 mL; 1.60; 7.85
-
-




