This is “Trace Elements in Biological Systems”, section 7.5 from the book Principles of General Chemistry (v. 1.0M). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
7.5 Trace Elements in Biological Systems
Learning Objective
- To describe some of the roles of trace elements in biological systems.
Of the more than 100 known elements, approximately 28 are known to be essential for the growth of at least one biological species, and only 19 are essential to humans. (For more information on essential elements, see Chapter 1 "Introduction to Chemistry", Section 1.8 "Essential Elements for Life", and Figure 1.26 "The Essential Elements in the Periodic Table".) What makes some elements essential to an organism and the rest nonessential? There are at least two reasons:
- The element must have some unique chemical property that an organism can use to its advantage and without which it cannot survive.
- Adequate amounts of the element must be available in the environment in an easily accessible form.
As you can see in Table 7.6 "Relative Abundance of Some Essential Elements in Earth’s Crust and Oceans", many of the elements that are abundant in Earth’s crust are nevertheless not found in an easily accessible form (e.g., as ions dissolved in seawater). Instead, they tend to form insoluble oxides, hydroxides, or carbonate salts. Although silicon is the second most abundant element in Earth’s crust, SiO2 and many silicate minerals are insoluble, so they are not easily absorbed by living tissues. This is also the case for iron and aluminum, which form insoluble hydroxides. Many organisms have therefore developed elaborate strategies to obtain iron from their environment. In contrast, molybdenum and iodine, though not particularly abundant, are highly soluble—molybdenum as molybdate (MoO42−) and iodine as iodide (I−) and iodate (IO3−)—and thus are more abundant in seawater than iron. Not surprisingly, both molybdenum and iodine are used by many organisms.
Table 7.6 Relative Abundance of Some Essential Elements in Earth’s Crust and Oceans
| Element* | Crust (ppm; average) | Seawater (mg/L = ppm) |
|---|---|---|
| O | 461,000 | 857,000 |
| Si | 282,000 | 2.2 |
| Al | 82,300 | 0.002 |
| Fe | 56,300 | 0.002 |
| Ca | 41,500 | 412 |
| Na | 23,600 | 10,800 |
| Mg | 23,300 | 1290 |
| K | 20,900 | 399 |
| H | 1400 | 108,000 |
| P | 1050 | 0.06 |
| Mn | 950 | 0.0002 |
| F | 585 | 1.3 |
| S | 350 | 905 |
| C | 200 | 28 |
| Cl | 145 | 19,400 |
| V | 120 | 0.0025 |
| Cr | 102 | 0.0003 |
| Ni | 84 | 0.00056 |
| Zn | 70 | 0.0049 |
| Cu | 60 | 0.00025 |
| Co | 25 | 0.00002 |
| Li | 20 | 0.18 |
| N | 19 | 0.5 |
| Br | 2.4 | 67.3 |
| Mo | 1.2 | 0.01 |
| I | 0.45 | 0.06 |
| Se | 0.05 | 0.0002 |
| *Elements in boldface are known to be essential to humans. | ||
Source: Data from CRC Handbook of Chemistry and Physics (2004).
Fortunately, many of the elements essential to life are necessary in only small amounts. (Table 1.6 "Approximate Elemental Composition of a Typical 70 kg Human" lists trace elements in humans.) Even so, elements that are present in trace amounts can exert large effects on the health of an organism. Such elements function as part of an amplification mechanismA process by which elements that are present in trace amouts can exert large effects on the health of an organism., in which a molecule containing a trace element is an essential part of a larger molecule that acts in turn to regulate the concentrations of other molecules, and so forth. The amplification mechanism enables small variations in the concentration of the trace element to have large biochemical effects.
Essential trace elementsElements that are required for the growth of most organisms. in mammals can have four general roles: (1) they can behave as macrominerals, (2) they can participate in the catalysis of group-transfer reactions, (3) they can participate in oxidation–reduction reactions, or (4) they can serve as structural components.
Macrominerals
The macromineralsAny of the six essential elements (Na, Mg, K, Ca, Cl, and P) that provide essential ions in body fluids and form the major structural components of the body.—Na, Mg, K, Ca, Cl, and P—are found in large amounts in biological tissues and are present as inorganic compounds, either dissolved or precipitated. All form monatomic ions (Na+, Mg2+, K+, Ca2+, Cl−) except for phosphorus, which is found as the phosphate ion (PO43−). Recall that calcium salts are used by many organisms as structural materials, such as in bone [hydroxyapatite, Ca5(PO4)3OH]; calcium salts are also in sea shells and egg shells (CaCO3), and they serve as a repository for Ca2+ in plants (calcium oxalate).
The body fluids of all multicellular organisms contain relatively high concentrations of these ions. Some ions (Na+, Ca2+, and Cl−) are located primarily in extracellular fluids such as blood plasma, whereas K+, Mg2+, and phosphate are located primarily in intracellular fluids. Substantial amounts of energy are required to selectively transport these ions across cell membranes. The selectivity of these ion pumpsA complex assembly of proteins that selectively transports ions across cell membranes toward the side with the higher concentration. is based on differences in ionic radius (Section 7.2 "Sizes of Atoms and Ions") and ionic charge.
Maintaining optimum levels of macrominerals is important because temporary changes in their concentration within a cell affect biological functions. For example, nerve impulse transmission requires a sudden, reversible increase in the amount of Na+ that flows into the nerve cell. Similarly, when hormones bind to a cell, they can cause Ca2+ ions to enter that cell. In a complex series of reactions, the Ca2+ ions trigger events such as muscle contraction, the release of neurotransmitters, or the secretion of hormones. When people who exercise vigorously for long periods of time overhydrate with water, low blood salt levels can result in a condition known as hyponatremia, which causes nausea, fatigue, weakness, seizures, and even death. For this reason, athletes should hydrate with a sports drink containing salts, not just water.
Group-Transfer Reactions
Trace metal ions also play crucial roles in many biological group-transfer reactionsA reaction in which a recognizable functional group is transferred from one molecule to another.. In these reactions, a recognizable functional group, such as a phosphoryl unit (−PO3−), is transferred from one molecule to another. In this example,
Equation 7.18
a unit is transferred from an alkoxide (RO−) to hydroxide (OH−). To neutralize the negative charge on the molecule that is undergoing the reaction, many biological reactions of this type require the presence of metal ions, such as Zn2+, Mn2+, Ca2+, or Mg2+ and occasionally Ni2+ or Fe3+. The effectiveness of the metal ion depends largely on its charge and radius.
Zinc is an important component of enzymes that catalyze the hydrolysis of proteins, the addition of water to CO2 to produce HCO3− and H+, and most of the reactions involved in DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) synthesis, repair, and replication. Consequently, zinc deficiency has serious adverse effects, including abnormal growth and sexual development and a loss of the sense of taste.
Biological Oxidation–Reduction Reactions
A third important role of trace elements is to transfer electrons in biological oxidation–reduction reactions. Iron and copper, for example, are found in proteins and enzymes that participate in O2 transport, the reduction of O2, the oxidation of organic molecules, and the conversion of atmospheric N2 to NH3. These metals usually transfer one electron per metal ion by alternating between oxidation states, such as 3+/2+ (Fe) or 2+/1+ (Cu).
Because most transition metals have multiple oxidation states separated by only one electron, they are uniquely suited to transfer multiple electrons one at a time. Examples include molybdenum (+6/+5/+4), which is widely used for two-electron oxidation–reduction reactions, and cobalt (+3/+2/+1), which is found in vitamin B12. In contrast, many of the p-block elements are well suited for transferring two electrons at once. Selenium (+4/+2), for example, is found in the enzyme that catalyzes the oxidation of glutathione (GSH) to its disulfide form (GSSG): 2 GSH + H2O2 → 2 H2O + GSSG. (For more information about glutathione and its disulfide form, see Chapter 4 "Reactions in Aqueous Solution", Section 4.9 "Quantitative Analysis Using Titrations", Example 20.)
Structural Components
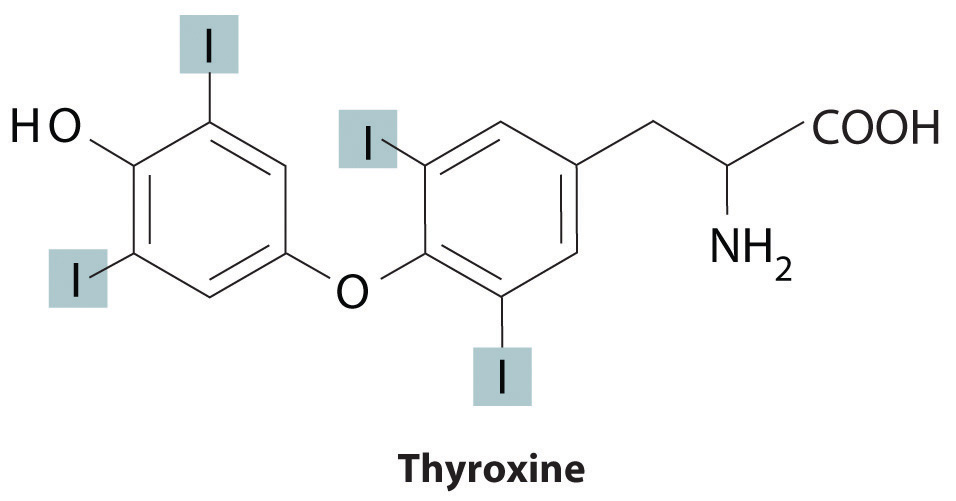
Trace elements also act as essential structural components of biological tissues or molecules. In many systems where trace elements do not change oxidation states or otherwise participate directly in biochemical reactions, it is often assumed, though frequently with no direct evidence, that the element stabilizes a particular three-dimensional structure of the biomolecule in which it is found. One example is a sugar-binding protein containing Mn2+ and Ca2+ that is a part of the biological defense system of certain plants. Other examples include enzymes that require Zn2+ at one site for activity to occur at a different site on the molecule. Some nonmetallic elements, such as F−, also appear to have structural roles. Fluoride, for example, displaces the hydroxide ion from hydroxyapatite in bone and teeth to form fluoroapatite [Ca5(PO4)3F]. Fluoroapatite is less soluble in acid and provides increased resistance to tooth decay. Another example of a nonmetal that plays a structural role is iodine, which in humans is found in only one molecule, the thyroid hormone thyroxine. When a person’s diet does not contain sufficient iodine, the thyroid glands in their neck become greatly enlarged, leading to a condition called goiter. Because iodine is found primarily in ocean fish and seaweed, many of the original settlers of the American Midwest developed goiter due to the lack of seafood in their diet. Today most table salt contains small amounts of iodine [actually potassium iodide (KI)] to prevent this problem.

An individual with goiter. In the United States, “iodized salt” prevents the occurrence of goiter.
Example 9
There is some evidence that tin is an essential element in mammals. Based solely on what you know about the chemistry of tin and its position in the periodic table, predict a likely biological function for tin.
Given: element and data in Table 1.6 "Approximate Elemental Composition of a Typical 70 kg Human"
Asked for: likely biological function
Strategy:
From the position of tin in the periodic table, its common oxidation states, and the data in Table 1.6 "Approximate Elemental Composition of a Typical 70 kg Human", predict a likely biological function for the element.
Solution:
From its position in the lower part of group 14, we know that tin is a metallic element whose most common oxidation states are +4 and +2. Given the low levels of tin in mammals (140 mg/70 kg human), tin is unlikely to function as a macromineral. Although a role in catalyzing group-transfer reactions or as an essential structural component cannot be ruled out, the most likely role for tin would be in catalyzing oxidation–reduction reactions that involve two-electron transfers. This would take advantage of the ability of tin to have two oxidation states separated by two electrons.
Exercise
Based solely on what you know about the chemistry of vanadium and its position in the periodic table, predict a likely biological function for vanadium.
Answer: Vanadium likely catalyzes oxidation–reduction reactions because it is a first-row transition metal and is likely to have multiple oxidation states.
Summary
Many of the elements in the periodic table are essential trace elements that are required for the growth of most organisms. Although they are present in only small quantities, they have important biological effects because of their participation in an amplification mechanism. Macrominerals are present in larger amounts and play structural roles or act as electrolytes whose distribution in cells is tightly controlled. These ions are selectively transported across cell membranes by ion pumps. Other trace elements catalyze group-transfer reactions or biological oxidation–reduction reactions, while others yet are essential structural components of biological molecules.
Key Takeaway
- Essential trace elements in mammals have four general roles: as macrominerals, as catalysts in group-transfer reactions or redox reactions, or as structural components.
Conceptual Problems
-
Give at least one criterion for essential elements involved in biological oxidation–reduction reactions. Which region of the periodic table contains elements that are very well suited for this role? Explain your reasoning.
-
What are the general biological roles of trace elements that do not have two or more accessible oxidation states?




