This is “End-of-Chapter Material”, section 2.7 from the book Principles of General Chemistry (v. 1.0M). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
2.7 End-of-Chapter Material
Application Problems
-
Carbon tetrachloride (CCl4) was used as a dry cleaning solvent until it was found to cause liver cancer. Based on the structure of chloroform given in Section 2.1 "Chemical Compounds", draw the structure of carbon tetrachloride.
-
Ammonium nitrate and ammonium sulfate are used in fertilizers as a source of nitrogen. The ammonium cation is tetrahedral. Refer to Section 2.1 "Chemical Compounds" to draw the structure of the ammonium ion.
-
The white light in fireworks displays is produced by burning magnesium in air, which contains oxygen. What compound is formed?
-
Sodium hydrogen sulfite, which is used for bleaching and swelling leather and to preserve flavor in almost all commercial wines, is made from sulfur dioxide. What are the formulas for these two sulfur-containing compounds?
-
Carbonic acid is used in carbonated drinks. When combined with lithium hydroxide, it produces lithium carbonate, a compound used to increase the brightness of pottery glazes and as a primary treatment for depression and bipolar disorder. Write the formula for both of these carbon-containing compounds.
-
Vinegar is a dilute solution of acetic acid, an organic acid, in water. What grouping of atoms would you expect to find in the structural formula for acetic acid?
-
♦ Sodamide, or sodium amide, is prepared from sodium metal and gaseous ammonia. Sodamide contains the amide ion (NH2−), which reacts with water to form the hydroxide anion by removing an H+ ion from water. Sodium amide is also used to prepare sodium cyanide.
- Write the formula for each of these sodium-containing compounds.
- What are the products of the reaction of sodamide with water?
-
A mixture of isooctane, n-pentane, and n-heptane is known to have an octane rating of 87. Use the data in Figure 2.25 "The Octane Ratings of Some Hydrocarbons and Common Additives" to calculate how much isooctane and n-heptane are present if the mixture is known to contain 30% n-pentane.
-
A crude petroleum distillate consists of 60% n-pentane, 25% methanol, and the remainder n-hexane by mass (Figure 2.25 "The Octane Ratings of Some Hydrocarbons and Common Additives").
- What is the octane rating?
- How much MTBE would have to be added to increase the octane rating to 93?
-
Premium gasoline sold in much of the central United States has an octane rating of 93 and contains 10% ethanol. What is the octane rating of the gasoline fraction before ethanol is added? (See Figure 2.25 "The Octane Ratings of Some Hydrocarbons and Common Additives".)
Problems marked with a ♦ involve multiple concepts.
Answers
-
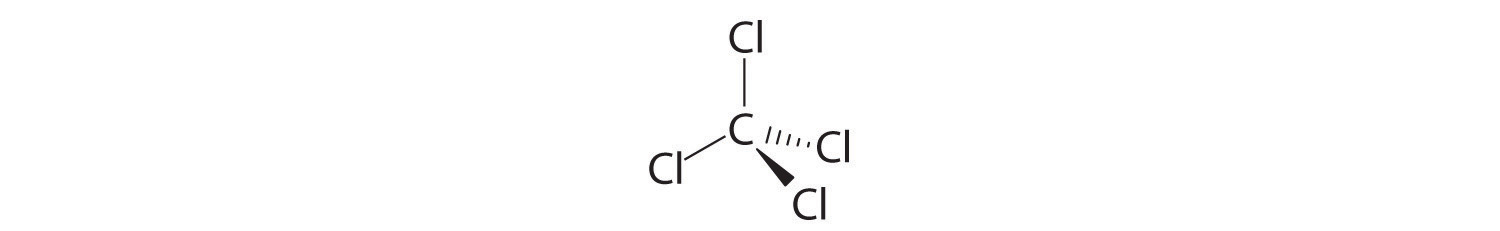
-
-
MgO, magnesium oxide
-
-
Carbonic acid is H2CO3; lithium carbonate is Li2CO3.
-
-
- Sodamide is NaNH2, and sodium cyanide is NaCN.
- Sodium hydroxide (NaOH) and ammonia (NH3).
-
-
- 68
- 52 g of MTBE must be added to 48 g of the crude distillate.
-




