This is “A Brief History of Chemistry”, section 1.4 from the book Principles of General Chemistry (v. 1.0M). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
1.4 A Brief History of Chemistry
Learning Objective
- To understand the development of the atomic model.
It was not until the era of the ancient Greeks that we have any record of how people tried to explain the chemical changes they observed and used. At that time, natural objects were thought to consist of only four basic elements: earth, air, fire, and water. Then, in the fourth century BC, two Greek philosophers, Democritus and Leucippus, suggested that matter was not infinitely divisible into smaller particles but instead consisted of fundamental, indivisible particles called atomsThe fundamental, individual particles of which matter is composed.. Unfortunately, these early philosophers did not have the technology to test their hypothesis. They would have been unlikely to do so in any case because the ancient Greeks did not conduct experiments or use the scientific method. They believed that the nature of the universe could be discovered by rational thought alone.
Over the next two millennia, alchemists, who engaged in a form of chemistry and speculative philosophy during the Middle Ages and Renaissance, achieved many advances in chemistry. Their major goal was to convert certain elements into others by a process they called transmutationThe process of converting one element to another. (Figure 1.13 "An Alchemist at Work"). In particular, alchemists wanted to find a way to transform cheaper metals into gold. Although most alchemists did not approach chemistry systematically and many appear to have been outright frauds, alchemists in China, the Arab kingdoms, and medieval Europe made major contributions, including the discovery of elements such as quicksilver (mercury) and the preparation of several strong acids.
Figure 1.13 An Alchemist at Work

Alchemy was a form of chemistry that flourished during the Middle Ages and Renaissance. Although some alchemists were frauds, others made major contributions, including the discovery of several elements and the preparation of strong acids.
Modern Chemistry
The 16th and 17th centuries saw the beginnings of what we now recognize as modern chemistry. During this period, great advances were made in metallurgy, the extraction of metals from ores, and the first systematic quantitative experiments were carried out. In 1661, the Englishman Robert Boyle (1627–91) published The Sceptical Chymist, which described the relationship between the pressure and the volume of air. More important, Boyle defined an element as a substance that cannot be broken down into two or more simpler substances by chemical means. This led to the identification of a large number of elements, many of which were metals. Ironically, Boyle himself never thought that metals were elements.
In the 18th century, the English clergyman Joseph Priestley (1733–1804) discovered oxygen gas and found that many carbon-containing materials burn vigorously in an oxygen atmosphere, a process called combustionThe burning of a material in an oxygen atmosphere.. Priestley also discovered that the gas produced by fermenting beer, which we now know to be carbon dioxide, is the same as one of the gaseous products of combustion. Priestley’s studies of this gas did not continue as he would have liked, however. After he fell into a vat of fermenting beer, brewers prohibited him from working in their factories. Although Priestley did not understand its identity, he found that carbon dioxide dissolved in water to produce seltzer water. In essence, he may be considered the founder of the multibillion-dollar carbonated soft drink industry.
Joseph Priestley (1733–1804)
Priestley was a political theorist and a leading Unitarian minister. He was appointed to Warrington Academy in Lancashire, England, where he developed new courses on history, science, and the arts. During visits to London, Priestley met the leading men of science, including Benjamin Franklin, who encouraged Priestley’s interest in electricity. Priestley’s work on gases began while he was living next to a brewery in Leeds, where he noticed “fixed air” bubbling out of vats of fermenting beer and ale. His scientific discoveries included the relationship between electricity and chemical change, 10 new “airs,” and observations that led to the discovery of photosynthesis. Due to his support for the principles of the French Revolution, Priestley’s house, library, and laboratory were destroyed by a mob in 1791. He and his wife emigrated to the United States in 1794 to join their three sons, who had previously emigrated to Pennsylvania. Priestley never returned to England and died in his new home in Pennsylvania.
Despite the pioneering studies of Priestley and others, a clear understanding of combustion remained elusive. In the late 18th century, however, the French scientist Antoine Lavoisier (1743–94) showed that combustion is the reaction of a carbon-containing substance with oxygen to form carbon dioxide and water and that life depends on a similar reaction, which today we call respiration. Lavoisier also wrote the first modern chemistry text and is widely regarded as the father of modern chemistry. His most important contribution was the law of conservation of massIn any chemical reaction, the mass of the substances that react equals the mass of the products that are formed., which states that in any chemical reaction, the mass of the substances that react equals the mass of the products that are formed. That is, in a chemical reaction, mass is neither lost nor destroyed. Unfortunately, Lavoisier invested in a private corporation that collected taxes for the Crown, and royal tax collectors were not popular during the French Revolution. He was executed on the guillotine at age 51, prematurely terminating his contributions to chemistry.
The Atomic Theory of Matter
In 1803, the English schoolteacher John Dalton (1766–1844) expanded Proust’s development of the law of definite proportions (Section 1.2 "The Scientific Method") and Lavoisier’s findings on the conservation of mass in chemical reactions to propose that elements consist of indivisible particles that he called atoms (taking the term from Democritus and Leucippus). Dalton’s atomic theory of matter contains four fundamental hypotheses:
- All matter is composed of tiny indivisible particles called atoms.
- All atoms of an element are identical in mass and chemical properties, whereas atoms of different elements differ in mass and fundamental chemical properties.
- A chemical compound is a substance that always contains the same atoms in the same ratio.
- In chemical reactions, atoms from one or more compounds or elements redistribute or rearrange in relation to other atoms to form one or more new compounds. Atoms themselves do not undergo a change of identity in chemical reactions.
This last hypothesis suggested that the alchemists’ goal of transmuting other elements to gold was impossible, at least through chemical reactions. We now know that Dalton’s atomic theory is essentially correct, with four minor modifications:
- Not all atoms of an element must have precisely the same mass.
- Atoms of one element can be transformed into another through nuclear reactions.
- The compositions of many solid compounds are somewhat variable.
- Under certain circumstances, some atoms can be divided (split into smaller particles).
These modifications illustrate the effectiveness of the scientific method; later experiments and observations were used to refine Dalton’s original theory.
The Law of Multiple Proportions
Despite the clarity of his thinking, Dalton could not use his theory to determine the elemental compositions of chemical compounds because he had no reliable scale of atomic masses; that is, he did not know the relative masses of elements such as carbon and oxygen. For example, he knew that the gas we now call carbon monoxide contained carbon and oxygen in the ratio 1:1.33 by mass, and a second compound, the gas we call carbon dioxide, contained carbon and oxygen in the ratio 1:2.66 by mass. Because 2.66/1.33 = 2.00, the second compound contained twice as many oxygen atoms per carbon atom as did the first. But what was the correct formula for each compound? If the first compound consisted of particles that contain one carbon atom and one oxygen atom, the second must consist of particles that contain one carbon atom and two oxygen atoms. If the first compound had two carbon atoms and one oxygen atom, the second must have two carbon atoms and two oxygen atoms. If the first had one carbon atom and two oxygen atoms, the second would have one carbon atom and four oxygen atoms, and so forth. Dalton had no way to distinguish among these or more complicated alternatives. However, these data led to a general statement that is now known as the law of multiple proportionsWhen two elements form a series of compounds, the ratios of the masses of the second element that are present per gram of the first element can almost always be expressed as the ratios of integers. (The same law holds for the mass ratios of compounds forming a series that contains more than two elements.): when two elements form a series of compounds, the ratios of the masses of the second element that are present per gram of the first element can almost always be expressed as the ratios of integers. (The same law holds for mass ratios of compounds forming a series that contains more than two elements.) Example 4 shows how the law of multiple proportions can be applied to determine the identity of a compound.
Example 4
A chemist is studying a series of simple compounds of carbon and hydrogen. The following table lists the masses of hydrogen that combine with 1 g of carbon to form each compound.
| Compound | Mass of Hydrogen (g) |
|---|---|
| A | 0.0839 |
| B | 0.1678 |
| C | 0.2520 |
| D |
- Determine whether these data follow the law of multiple proportions.
- Calculate the mass of hydrogen that would combine with 1 g of carbon to form D, the fourth compound in the series.
Given: mass of hydrogen per gram of carbon for three compounds
Asked for:
- ratios of masses of hydrogen to carbon
- mass of hydrogen per gram of carbon for fourth compound in series
Strategy:
A Select the lowest mass to use as the denominator and then calculate the ratio of each of the other masses to that mass. Include other ratios if appropriate.
B If the ratios are small whole integers, the data follow the law of multiple proportions.
C Decide whether the ratios form a numerical series. If so, then determine the next member of that series and predict the ratio corresponding to the next compound in the series.
D Use proportions to calculate the mass of hydrogen per gram of carbon in that compound.
Solution:
A Compound A has the lowest mass of hydrogen, so we use it as the denominator. The ratios of the remaining masses of hydrogen, B and C, that combine with 1 g of carbon are as follows:
B The ratios of the masses of hydrogen that combine with 1 g of carbon are indeed composed of small whole integers (3/1, 2/1, 3/2), as predicted by the law of multiple proportions.
C The ratios B/A and C/A form the series 2/1, 3/1, so the next member of the series should be D/A = 4/1.
D Thus, if compound D exists, it would be formed by combining 4 × 0.0839 g = 0.336 g of hydrogen with 1 g of carbon. Such a compound does exist; it is methane, the major constituent of natural gas.
Exercise
Four compounds containing only sulfur and fluorine are known. The following table lists the masses of fluorine that combine with 1 g of sulfur to form each compound.
| Compound | Mass of Fluorine (g) |
|---|---|
| A | 3.54 |
| B | 2.96 |
| C | 2.36 |
| D | 0.59 |
- Determine the ratios of the masses of fluorine that combine with 1 g of sulfur in these compounds. Are these data consistent with the law of multiple proportions?
- Calculate the mass of fluorine that would combine with 1 g of sulfur to form the next two compounds in the series: E and F.
Answer:
- A/D = 6.0 or 6/1; B/D ≈ 5.0, or 5/1; C/D = 4.0, or 4/1; yes
- Ratios of 3.0 and 2.0 give 1.8 g and 1.2 g of fluorine/gram of sulfur, respectively. (Neither of these compounds is yet known.)
Avogadro’s Hypothesis
In a further attempt to establish the formulas of chemical compounds, the French chemist Joseph Gay-Lussac (1778–1850) carried out a series of experiments using volume measurements. Under conditions of constant temperature and pressure, he carefully measured the volumes of gases that reacted to make a given chemical compound, together with the volumes of the products if they were gases. Gay-Lussac found, for example, that one volume of chlorine gas always reacted with one volume of hydrogen gas to produce two volumes of hydrogen chloride gas. Similarly, one volume of oxygen gas always reacted with two volumes of hydrogen gas to produce two volumes of water vapor (part (a) in Figure 1.14 "Gay-Lussac’s Experiments with Chlorine Gas and Hydrogen Gas").
Figure 1.14 Gay-Lussac’s Experiments with Chlorine Gas and Hydrogen Gas
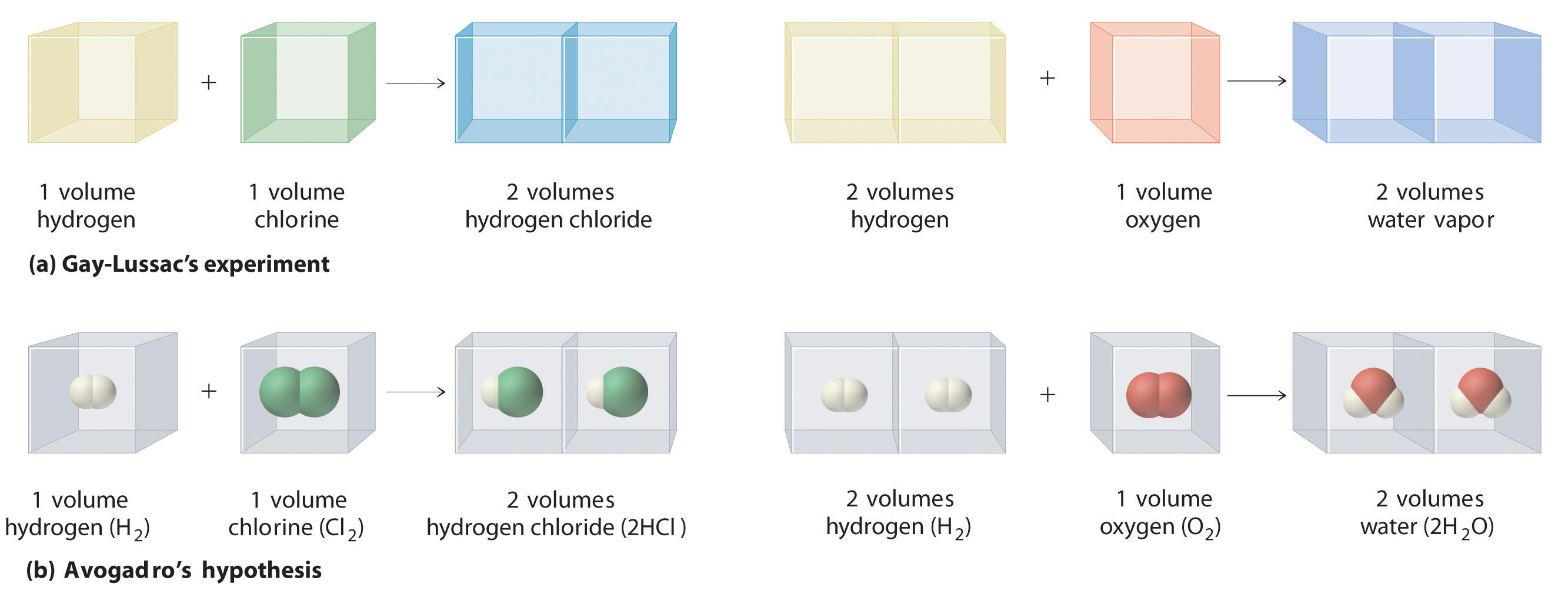
(a) One volume of chlorine gas reacted with one volume of hydrogen gas to produce two volumes of hydrogen chloride gas, and one volume of oxygen gas reacted with two volumes of hydrogen gas to produce two volumes of water vapor. (b) A summary of Avogadro’s hypothesis, which interpreted Gay-Lussac’s results in terms of atoms. Note that the simplest way for two molecules of hydrogen chloride to be produced is if hydrogen and chlorine each consist of molecules that contain two atoms of the element.
Gay-Lussac’s results did not by themselves reveal the formulas for hydrogen chloride and water. The Italian chemist Amadeo Avogadro (1776–1856) developed the key insight that led to the exact formulas. He proposed that when gases are measured at the same temperature and pressure, equal volumes of different gases contain equal numbers of gas particles. Avogadro’s hypothesis, which explained Gay-Lussac’s results, is summarized here and in part (b) in Figure 1.14 "Gay-Lussac’s Experiments with Chlorine Gas and Hydrogen Gas":
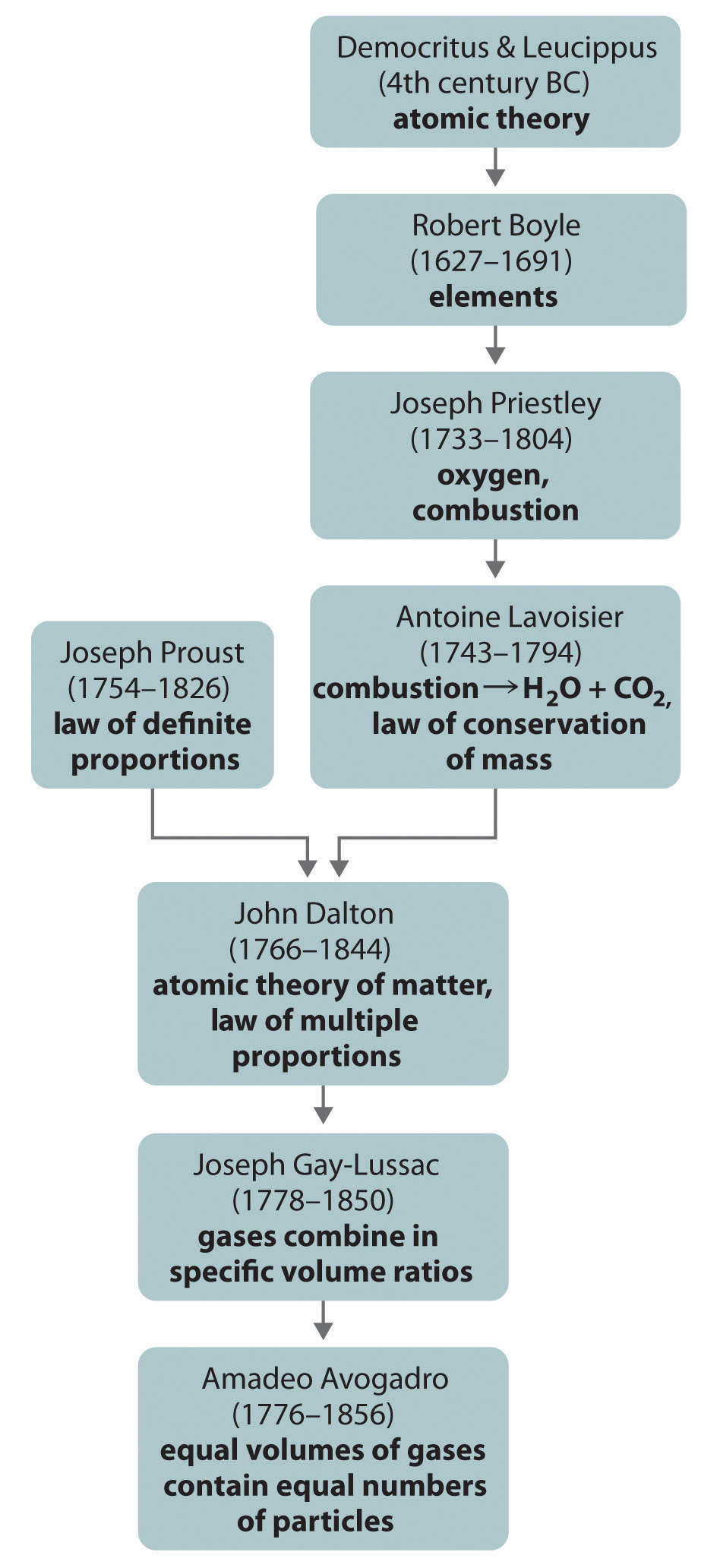
If Dalton’s theory of atoms was correct, then each particle of hydrogen or chlorine had to contain at least two atoms of hydrogen or chlorine because two particles of hydrogen chloride were produced. The simplest—but not the only—explanation was that hydrogen and chlorine contained two atoms each (i.e., they were diatomic) and that hydrogen chloride contained one atom each of hydrogen and chlorine. Applying this reasoning to Gay-Lussac’s results with hydrogen and oxygen leads to the conclusion that water contains two hydrogen atoms per oxygen atom. Unfortunately, because no data supported Avogadro’s hypothesis that equal volumes of gases contained equal numbers of particles, his explanations and formulas for simple compounds were not generally accepted for more than 50 years. Dalton and many others continued to believe that water particles contained one hydrogen atom and one oxygen atom, rather than two hydrogen atoms and one oxygen atom. The historical development of the concept of the atom is summarized in Figure 1.15 "A Summary of the Historical Development of the Concept of the Atom".
Figure 1.15 A Summary of the Historical Development of the Concept of the Atom
Summary
The ancient Greeks first proposed that matter consisted of fundamental particles called atoms. Chemistry took its present scientific form in the 18th century, when careful quantitative experiments by Lavoisier, Proust, and Dalton resulted in the law of definite proportions, the law of conservation of mass, and the law of multiple proportions, which laid the groundwork for Dalton’s atomic theory of matter. In particular, Avogadro’s hypothesis provided the first link between the macroscopic properties of a substance (in this case, the volume of a gas) and the number of atoms or molecules present.
Key Takeaway
- The development of the atomic model relied on the application of the scientific method over several centuries.
Conceptual Problems
-
Define combustion and discuss the contributions made by Priestley and Lavoisier toward understanding a combustion reaction.
-
Chemical engineers frequently use the concept of “mass balance” in their calculations, in which the mass of the reactants must equal the mass of the products. What law supports this practice?
-
Does the law of multiple proportions apply to both mass ratios and atomic ratios? Why or why not?
-
What are the four hypotheses of the atomic theory of matter?
-
Much of the energy in France is provided by nuclear reactions. Are such reactions consistent with Dalton’s hypotheses? Why or why not?
-
Does 1 L of air contain the same number of particles as 1 L of nitrogen gas? Explain your answer.
Numerical Problems
-
One of the minerals found in soil has an Al:Si:O atomic ratio of 0.2:0.2:0.5. Is this consistent with the law of multiple proportions? Why or why not? Is the ratio of elements consistent with Dalton’s atomic theory of matter?
-
Nitrogen and oxygen react to form three different compounds that contain 0.571 g, 1.143 g, and 2.285 g of oxygen/gram of nitrogen, respectively. Is this consistent with the law of multiple proportions? Explain your answer.
-
Three binary compounds of vanadium and oxygen are known. The following table gives the masses of oxygen that combine with 10.00 g of vanadium to form each compound.
Compound Mass of Oxygen (g) A 4.71 B 6.27 C - Determine the ratio of the masses of oxygen that combine with 3.14 g of vanadium in compounds A and B.
- Predict the mass of oxygen that would combine with 3.14 g of vanadium to form the third compound in the series.
-
Three compounds containing titanium, magnesium, and oxygen are known. The following table gives the masses of titanium and magnesium that react with 5.00 g of oxygen to form each compound.
Compound Mass of Titanium (g) Mass of Magnesium (g) A 4.99 2.53 B 3.74 3.80 C - Determine the ratios of the masses of titanium and magnesium that combine with 5.00 g of oxygen in these compounds.
- Predict the masses of titanium and magnesium that would combine with 5.00 g of oxygen to form another possible compound in the series: C.
Please be sure you are familiar with the topics discussed in Essential Skills 1 (Section 1.9 "Essential Skills 1") before proceeding to the Numerical Problems.




