This is “Stage III of Catabolism”, section 20.4 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
20.4 Stage III of Catabolism
Learning Objectives
- Describe the reactions of the citric acid cycle.
- Describe the function of the citric acid cycle and identify the products produced.
- Describe the role of the electron transport chain in energy metabolism.
- Describe the role of oxidative phosphorylation in energy metabolism.
The acetyl group enters a cyclic sequence of reactions known collectively as the citric acid cycle (or Krebs cycle or tricarboxylic acid [TCA] cycle)A cyclic sequence of reactions that brings about the oxidation of a two-C unit to carbon dioxide and water.. The cyclical design of this complex series of reactions, which bring about the oxidation of the acetyl group of acetyl-CoA to carbon dioxide and water, was first proposed by Hans Krebs in 1937. (He was awarded the 1953 Nobel Prize in Physiology or Medicine.) Acetyl-CoA’s entrance into the citric acid cycle is the beginning of stage III of catabolism. The citric acid cycle produces adenosine triphosphate (ATP), reduced nicotinamide adenine dinucleotide (NADH), reduced flavin adenine dinucleotide (FADH2), and metabolic intermediates for the synthesis of needed compounds.
Steps of the Citric Acid Cycle
At first glance, the citric acid cycle appears rather complex (Figure 20.12 "Reactions of the Citric Acid Cycle"). All the reactions, however, are familiar types in organic chemistry: hydration, oxidation, decarboxylation, and hydrolysis. Each reaction of the citric acid cycle is numbered, and in Figure 20.12 "Reactions of the Citric Acid Cycle", the two acetyl carbon atoms are highlighted in red. Each intermediate in the cycle is a carboxylic acid, existing as an anion at physiological pH. All the reactions occur within the mitochondria, which are small organelles within the cells of plants and animals. We will look more closely at the structure of mitochondria in Section 20.5 "Stage II of Carbohydrate Catabolism".
Figure 20.12 Reactions of the Citric Acid Cycle
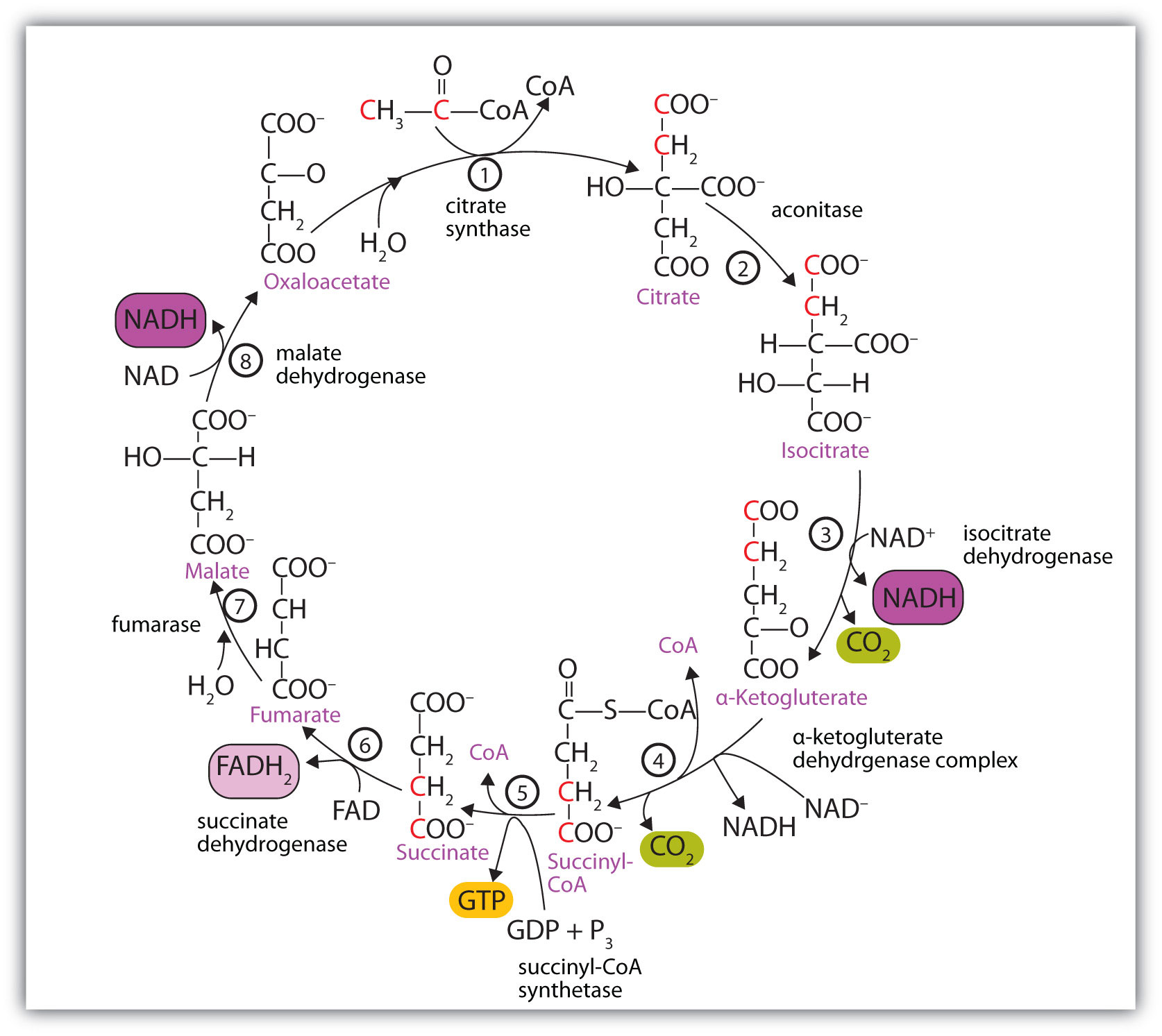
In the first reaction, acetyl-CoA enters the citric acid cycle, and the acetyl group is transferred onto oxaloacetate, yielding citrate. Note that this step releases coenzyme A. The reaction is catalyzed by citrate synthase.
In the next step, aconitase catalyzes the isomerization of citrate to isocitrate. In this reaction, a tertiary alcohol, which cannot be oxidized, is converted to a secondary alcohol, which can be oxidized in the next step.
Isocitrate then undergoes a reaction known as oxidative decarboxylation because the alcohol is oxidized and the molecule is shortened by one carbon atom with the release of carbon dioxide (decarboxylation). The reaction is catalyzed by isocitrate dehydrogenase, and the product of the reaction is α-ketoglutarate. An important reaction linked to this is the reduction of the coenzyme nicotinamide adenine dinucleotide (NAD+) to NADH. The NADH is ultimately reoxidized, and the energy released is used in the synthesis of ATP, as we shall see.
The fourth step is another oxidative decarboxylation. This time α-ketoglutarate is converted to succinyl-CoA, and another molecule of NAD+ is reduced to NADH. The α-ketoglutarate dehydrogenase complex catalyzes this reaction. This is the only irreversible reaction in the citric acid cycle. As such, it prevents the cycle from operating in the reverse direction, in which acetyl-CoA would be synthesized from carbon dioxide.
Comment: So far, in the first four steps, two carbon atoms have entered the cycle as an acetyl group, and two carbon atoms have been released as molecules of carbon dioxide. The remaining reactions of the citric acid cycle use the four carbon atoms of the succinyl group to resynthesize a molecule of oxaloacetate, which is the compound needed to combine with an incoming acetyl group and begin another round of the cycle.
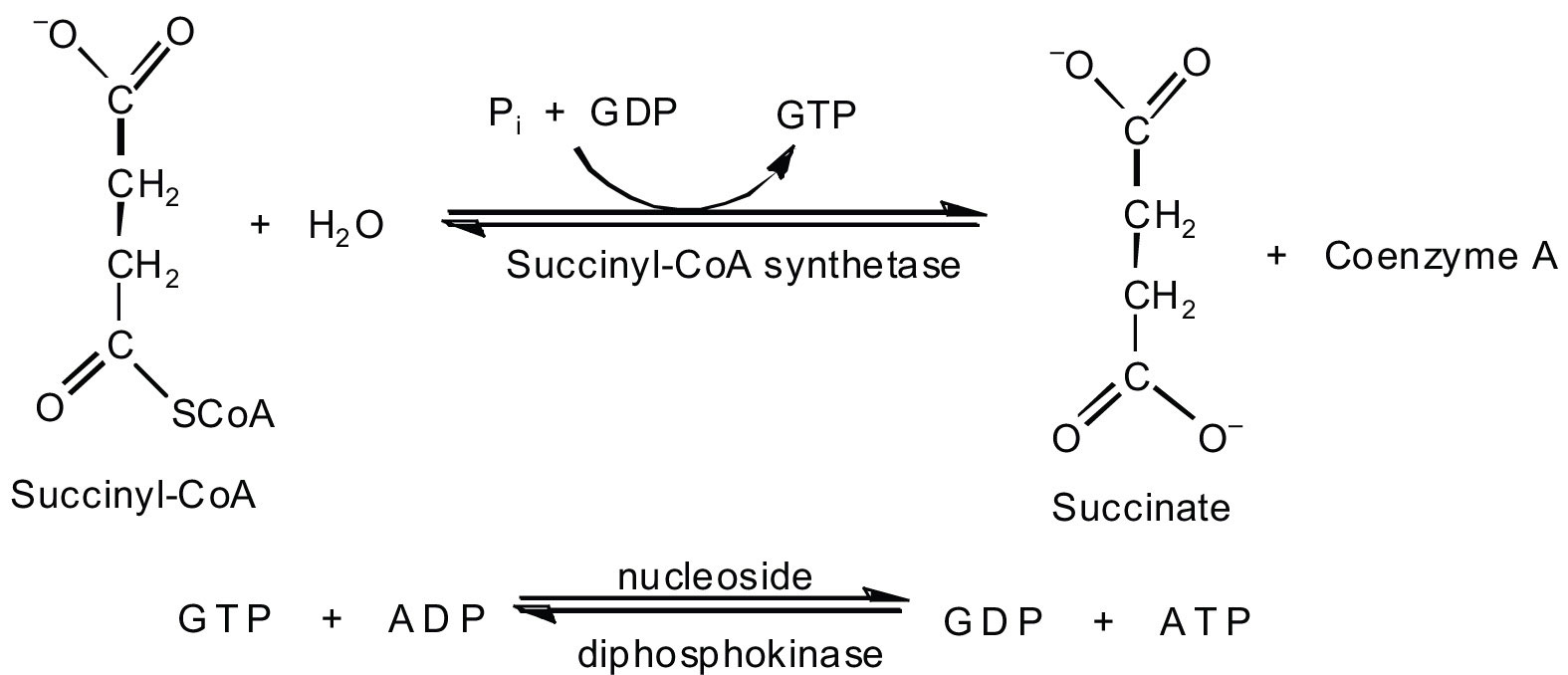
In the fifth reaction, the energy released by the hydrolysis of the high-energy thioester bond of succinyl-CoA is used to form guanosine triphosphate (GTP) from guanosine diphosphate (GDP) and inorganic phosphate in a reaction catalyzed by succinyl-CoA synthetase. This step is the only reaction in the citric acid cycle that directly forms a high-energy phosphate compound. GTP can readily transfer its terminal phosphate group to adenosine diphosphate (ADP) to generate ATP in the presence of nucleoside diphosphokinase.
Succinate dehydrogenase then catalyzes the removal of two hydrogen atoms from succinate, forming fumarate. This oxidation-reduction reaction uses flavin adenine dinucleotide (FAD), rather than NAD+, as the oxidizing agent. Succinate dehydrogenase is the only enzyme of the citric acid cycle located within the inner mitochondrial membrane. We will see soon the importance of this.
In the following step, a molecule of water is added to the double bond of fumarate to form L-malate in a reaction catalyzed by fumarase.
One revolution of the cycle is completed with the oxidation of L-malate to oxaloacetate, brought about by malate dehydrogenase. This is the third oxidation-reduction reaction that uses NAD+ as the oxidizing agent. Oxaloacetate can accept an acetyl group from acetyl-CoA, allowing the cycle to begin again.
Cellular Respiration
Respiration can be defined as the process by which cells oxidize organic molecules in the presence of gaseous oxygen to produce carbon dioxide, water, and energy in the form of ATP. We have seen that two carbon atoms enter the citric acid cycle from acetyl-CoA (step 1), and two different carbon atoms exit the cycle as carbon dioxide (steps 3 and 4). Yet nowhere in our discussion of the citric acid cycle have we indicated how oxygen is used. Recall, however, that in the four oxidation-reduction steps occurring in the citric acid cycle, the coenzyme NAD+ or FAD is reduced to NADH or FADH2, respectively. Oxygen is needed to reoxidize these coenzymes. Recall, too, that very little ATP is obtained directly from the citric acid cycle. Instead, oxygen participation and significant ATP production occur subsequent to the citric acid cycle, in two pathways that are closely linked: electron transport and oxidative phosphorylation.
All the enzymes and coenzymes for the citric acid cycle, the reoxidation of NADH and FADH2, and the production of ATP are located in the mitochondriaSmall, oval organelles with double membranes; the “power plants” of a cell., which are small, oval organelles with double membranes, often referred to as the “power plants” of the cell (Figure 20.13 "Respiration"). A cell may contain 100–5,000 mitochondria, depending on its function, and the mitochondria can reproduce themselves if the energy requirements of the cell increase.
Figure 20.13 Respiration
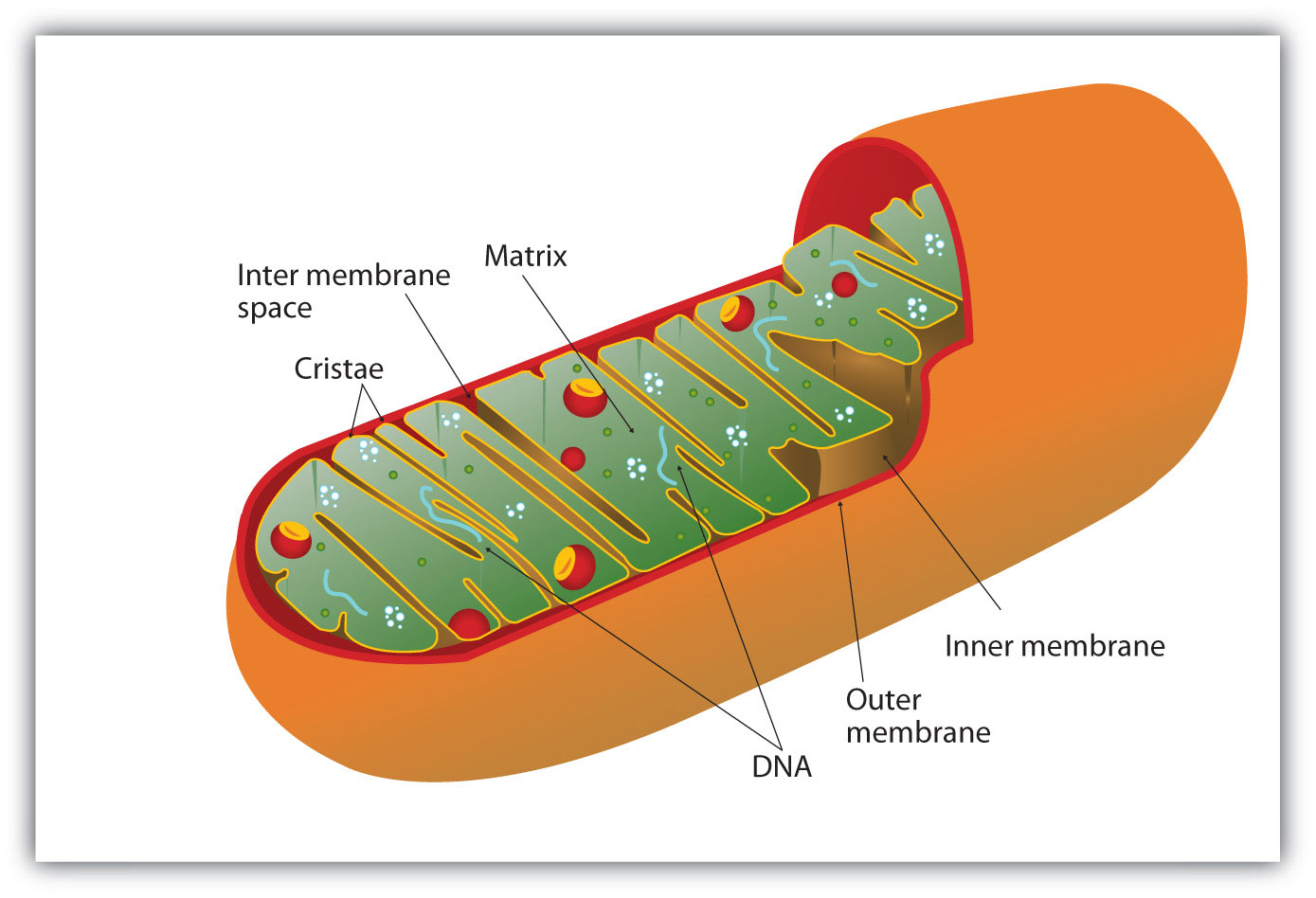
Cellular respiration occurs in the mitochondria.
Figure 20.13 "Respiration" shows the mitochondrion’s two membranes: outer and inner. The inner membrane is extensively folded into a series of internal ridges called cristae. Thus there are two compartments in mitochondria: the intermembrane space, which lies between the membranes, and the matrix, which lies inside the inner membrane. The outer membrane is permeable, whereas the inner membrane is impermeable to most molecules and ions, although water, oxygen, and carbon dioxide can freely penetrate both membranes. The matrix contains all the enzymes of the citric acid cycle with the exception of succinate dehydrogenase, which is embedded in the inner membrane. The enzymes that are needed for the reoxidation of NADH and FADH2 and ATP production are also located in the inner membrane. They are arranged in specific positions so that they function in a manner analogous to a bucket brigade. This highly organized sequence of oxidation-reduction enzymes is known as the electron transport chain (or respiratory chain)An organized sequence of oxidation-reduction reactions that ultimately transports electrons to oxygen, reducing it to water..
Electron Transport
Figure 20.14 "The Mitochondrial Electron Transport Chain and ATP Synthase" illustrates the organization of the electron transport chain. The components of the chain are organized into four complexes designated I, II, III, and IV. Each complex contains several enzymes, other proteins, and metal ions. The metal ions can be reduced and then oxidized repeatedly as electrons are passed from one component to the next. Recall from Chapter 5 "Introduction to Chemical Reactions", Section 5.5 "Oxidation-Reduction (Redox) Reactions", that a compound is reduced when it gains electrons or hydrogen atoms and is oxidized when it loses electrons or hydrogen atoms.
Figure 20.14 The Mitochondrial Electron Transport Chain and ATP Synthase
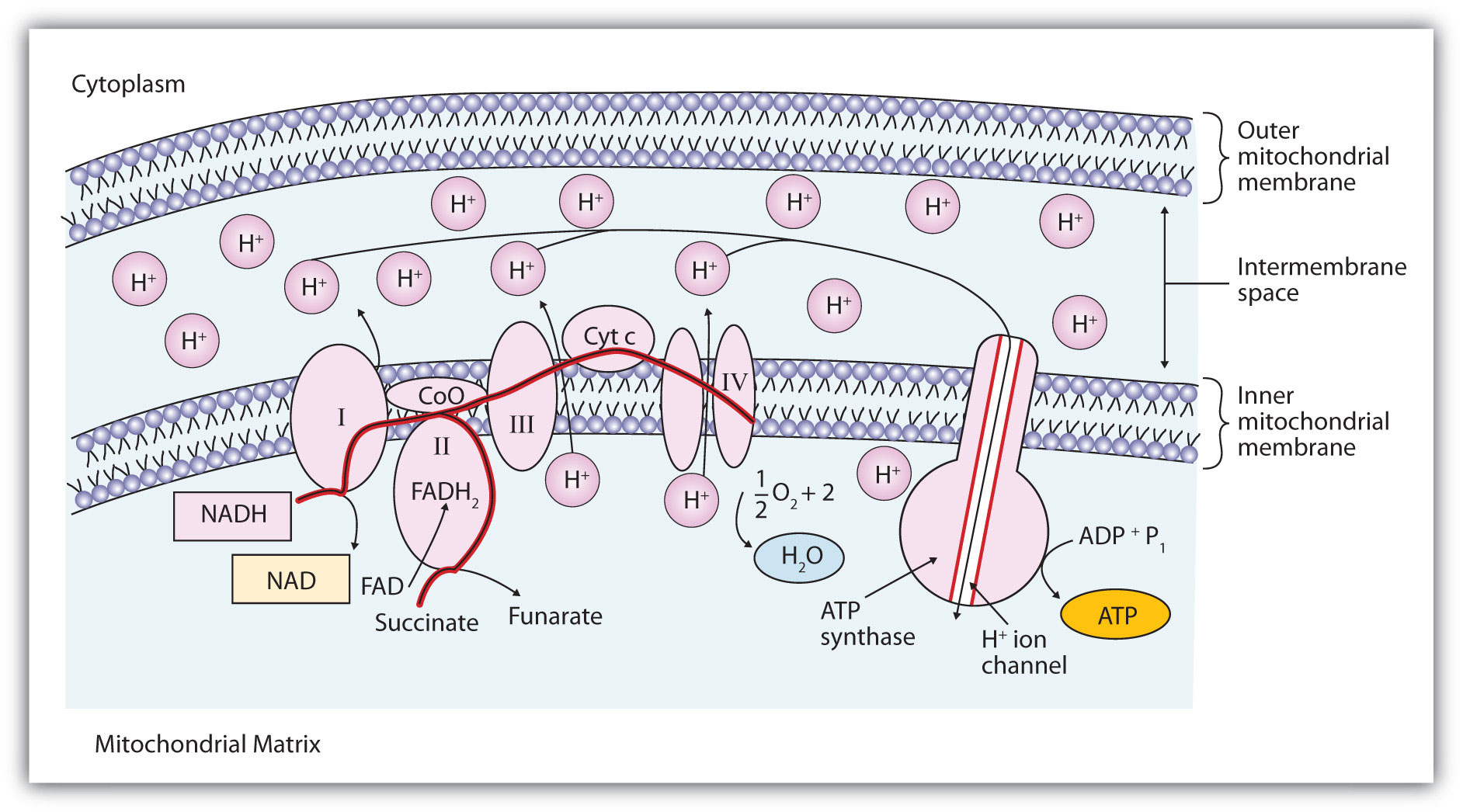
The red line shows the path of electrons.
Electrons can enter the electron transport chain through either complex I or II. We will look first at electrons entering at complex I. These electrons come from NADH, which is formed in three reactions of the citric acid cycle. Let’s use step 8 as an example, the reaction in which L-malate is oxidized to oxaloacetate and NAD+ is reduced to NADH. This reaction can be divided into two half reactions:
Oxidation half-reaction:
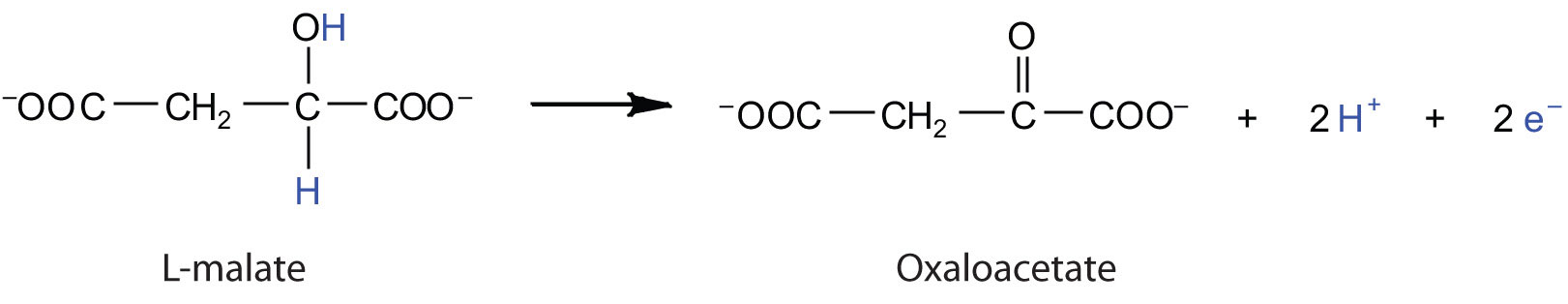
Reduction half-reaction:
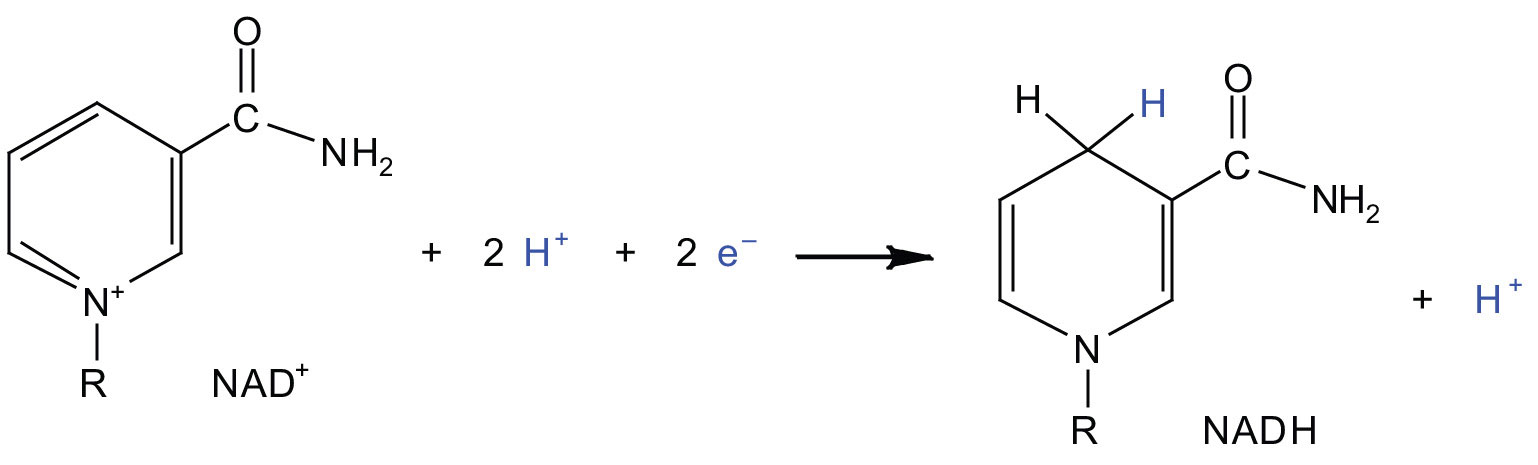
In the oxidation half-reaction, two hydrogen (H+) ions and two electrons are removed from the substrate. In the reduction half-reaction, the NAD+ molecule accepts both of those electrons and one of the H+ ions. The other H+ ion is transported from the matrix, across the inner mitochondrial membrane, and into the intermembrane space. The NADH diffuses through the matrix and is bound by complex I of the electron transport chain. In the complex, the coenzyme flavin mononucleotide (FMN) accepts both electrons from NADH. By passing the electrons along, NADH is oxidized back to NAD+ and FMN is reduced to FMNH2 (reduced form of flavin mononucleotide). Again, the reaction can be illustrated by dividing it into its respective half-reactions.
Oxidation half-reaction:
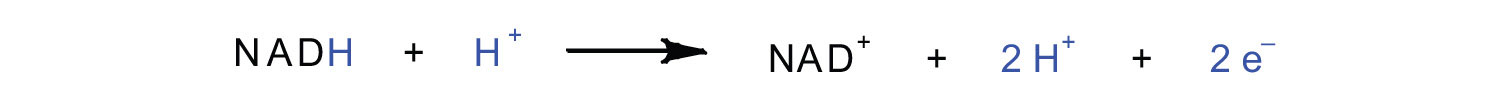
Reduction half-reaction:
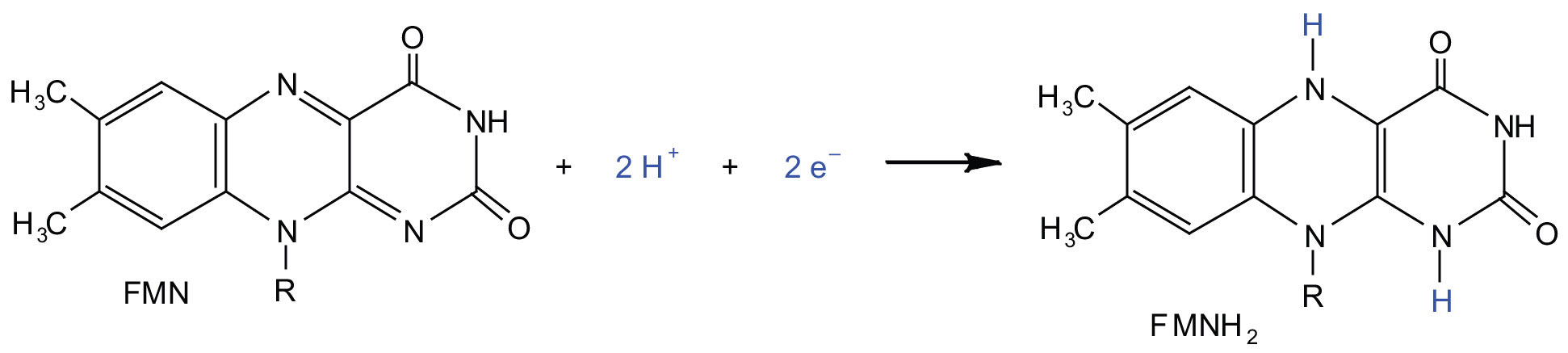
Complex I contains several proteins that have iron-sulfur (Fe·S) centers. The electrons that reduced FMN to FMNH2 are now transferred to these proteins. The iron ions in the Fe·S centers are in the Fe(III) form at first, but by accepting an electron, each ion is reduced to the Fe(II) form. Because each Fe·S center can transfer only one electron, two centers are needed to accept the two electrons that will regenerate FMN.
Oxidation half-reaction:
FMNH2 → FMN + 2H+ + 2e−Reduction half-reaction:
2Fe(III) · S + 2e− → 2Fe(II) · SElectrons from FADH2, formed in step 6 of the citric acid cycle, enter the electron transport chain through complex II. Succinate dehydrogenase, the enzyme in the citric acid cycle that catalyzes the formation of FADH2 from FAD is part of complex II. The electrons from FADH2 are then transferred to an Fe·S protein.
Oxidation half-reaction:
FADH2 → FAD + 2H+ + 2e−Reduction half-reaction:
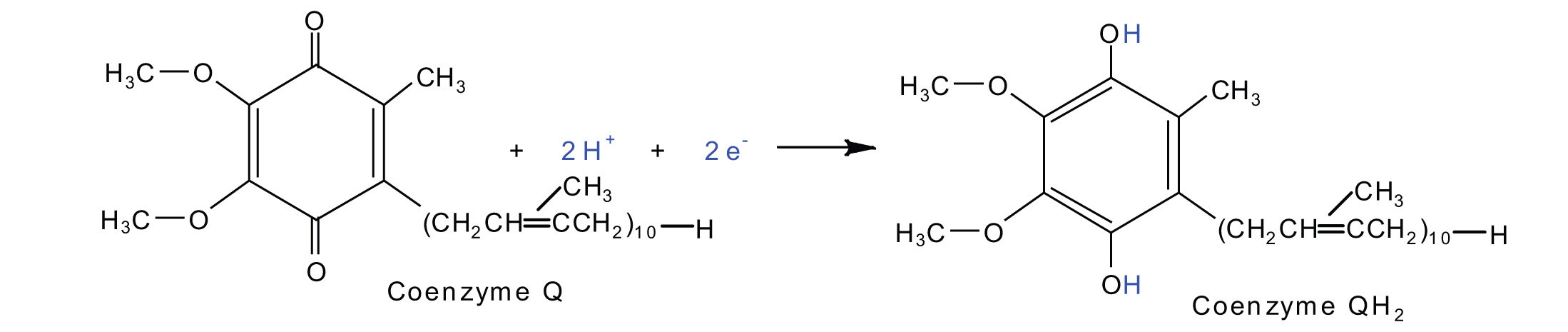
2Fe(III) · S + 2e− → 2Fe(II) · SElectrons from complexes I and II are then transferred from the Fe·S protein to coenzyme Q (CoQ), a mobile electron carrier that acts as the electron shuttle between complexes I or II and complex III.
Note
Coenzyme Q is also called ubiquinone because it is ubiquitous in living systems.
Oxidation half-reaction:
2Fe(II) · S → 2Fe(III) · S + 2e−Reduction half-reaction:
Complexes III and IV include several iron-containing proteins known as cytochromesA protein that contains an iron porphyrin in which iron can alternate between Fe(II) and Fe(III).. The iron in these enzymes is located in substructures known as iron porphyrins (Figure 20.15 "An Iron Porphyrin"). Like the Fe·S centers, the characteristic feature of the cytochromes is the ability of their iron atoms to exist as either Fe(II) or Fe(III). Thus, each cytochrome in its oxidized form—Fe(III)—can accept one electron and be reduced to the Fe(II) form. This change in oxidation state is reversible, so the reduced form can donate its electron to the next cytochrome, and so on. Complex III contains cytochromes b and c, as well as Fe·S proteins, with cytochrome c acting as the electron shuttle between complex III and IV. Complex IV contains cytochromes a and a3 in an enzyme known as cytochrome oxidase. This enzyme has the ability to transfer electrons to molecular oxygen, the last electron acceptor in the chain of electron transport reactions. In this final step, water (H2O) is formed.
Oxidation half-reaction:
4Cyt a3–Fe(II) → 4Cyt a3–Fe(III) + 4e−Reduction half-reaction:
O2 + 4H+ + 4e− → 2H2OFigure 20.15 An Iron Porphyrin
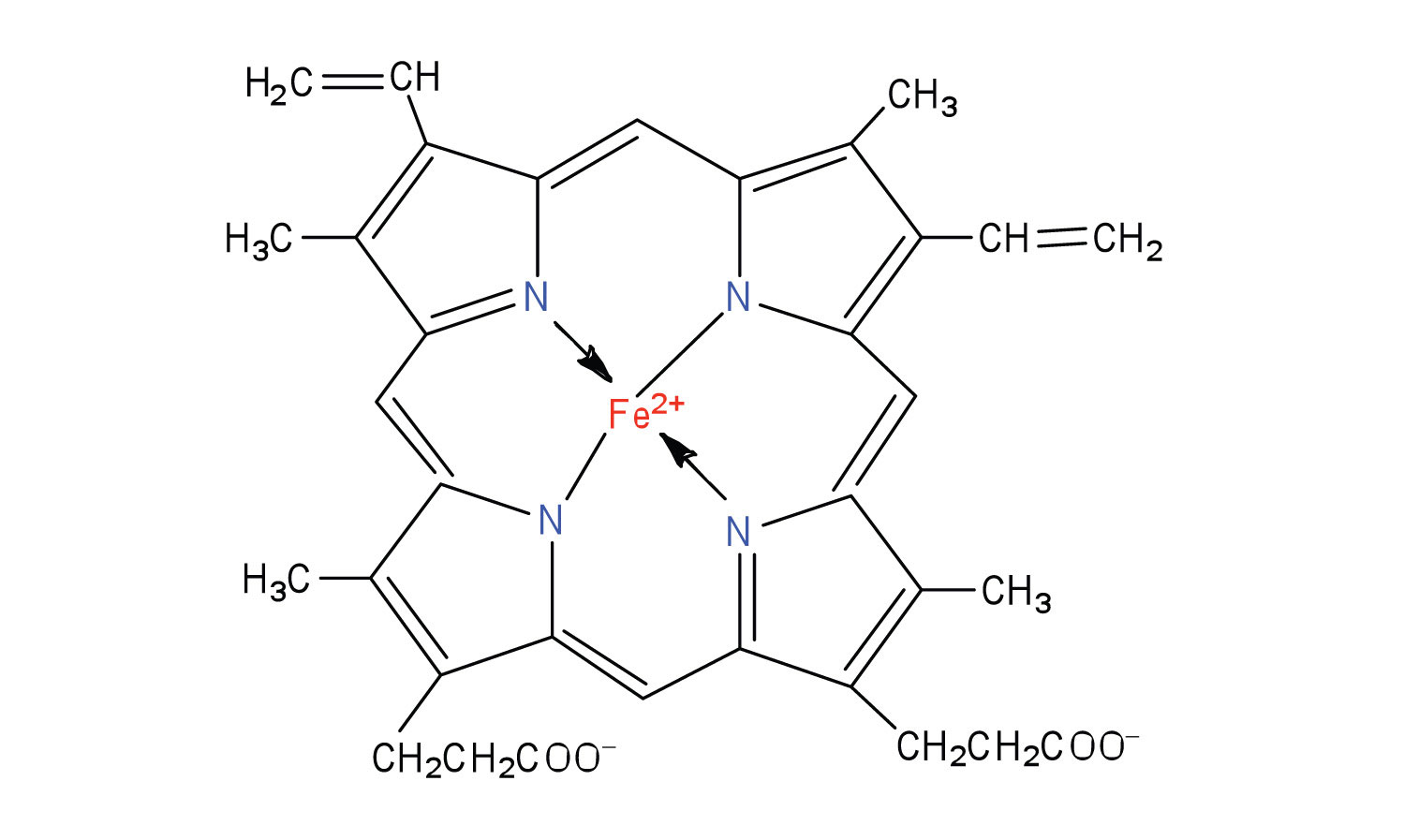
Iron porphyrins are present in cytochromes as well as in myoglobin and hemoglobin.
Oxidative Phosphorylation
Each intermediate compound in the electron transport chain is reduced by the addition of one or two electrons in one reaction and then subsequently restored to its original form by delivering the electron(s) to the next compound along the chain. The successive electron transfers result in energy production. But how is this energy used for the synthesis of ATP? The process that links ATP synthesis to the operation of the electron transport chain is referred to as oxidative phosphorylationThe process that links ATP synthesis to the operation of the electron transport chain..
Electron transport is tightly coupled to oxidative phosphorylation. The coenzymes NADH and FADH2 are oxidized by the respiratory chain only if ADP is simultaneously phosphorylated to ATP. The currently accepted model explaining how these two processes are linked is known as the chemiosmotic hypothesis, which was proposed by Peter Mitchell, resulting in Mitchell being awarded the 1978 Nobel Prize in Chemistry.
Looking again at Figure 20.14 "The Mitochondrial Electron Transport Chain and ATP Synthase", we see that as electrons are being transferred through the electron transport chain, hydrogen (H+) ions are being transported across the inner mitochondrial membrane from the matrix to the intermembrane space. The concentration of H+ is already higher in the intermembrane space than in the matrix, so energy is required to transport the additional H+ there. This energy comes from the electron transfer reactions in the electron transport chain. But how does the extreme difference in H+ concentration then lead to ATP synthesis? The buildup of H+ ions in the intermembrane space results in an H+ ion gradient that is a large energy source, like water behind a dam (because, given the opportunity, the protons will flow out of the intermembrane space and into the less concentrated matrix). Current research indicates that the flow of H+ down this concentration gradient through a fifth enzyme complex, known as ATP synthase, leads to a change in the structure of the synthase, causing the synthesis and release of ATP.
In cells that are using energy, the turnover of ATP is very high, so these cells contain high levels of ADP. They must therefore consume large quantities of oxygen continuously, so as to have the energy necessary to phosphorylate ADP to form ATP. Consider, for example, that resting skeletal muscles use about 30% of a resting adult’s oxygen consumption, but when the same muscles are working strenuously, they account for almost 90% of the total oxygen consumption of the organism.
Experiment has shown that 2.5–3 ATP molecules are formed for every molecule of NADH oxidized in the electron transport chain, and 1.5–2 ATP molecules are formed for every molecule of FADH2 oxidized. Table 20.2 "Maximum Yield of ATP from the Complete Oxidation of 1 Mol of Acetyl-CoA" summarizes the theoretical maximum yield of ATP produced by the complete oxidation of 1 mol of acetyl-CoA through the sequential action of the citric acid cycle, the electron transport chain, and oxidative phosphorylation.
Table 20.2 Maximum Yield of ATP from the Complete Oxidation of 1 Mol of Acetyl-CoA
| Reaction | Comments | Yield of ATP (moles) |
|---|---|---|
| Isocitrate → α-ketoglutarate + CO2 | produces 1 mol NADH | |
| α-ketoglutarate → succinyl-CoA + CO2 | produces 1 mol NADH | |
| Succinyl-CoA → succinate | produces 1 mol GTP | +1 |
| Succinate → fumarate | produces 1 mol FADH2 | |
| Malate → oxaloacetate | produces 1 mol NADH | |
| 1 FADH2 from the citric acid cycle | yields 2 mol ATP | +2 |
| 3 NADH from the citric acid cycle | yields 3 mol ATP/NADH | +9 |
| Net yield of ATP: | +12 | |
Concept Review Exercises
-
What is the main function of the citric acid cycle?
-
Two carbon atoms are fed into the citric acid cycle as acetyl-CoA. In what form are two carbon atoms removed from the cycle?
-
What are mitochondria and what is their function in the cell?
Answers
-
the complete oxidation of carbon atoms to carbon dioxide and the formation of a high-energy phosphate compound, energy rich reduced coenzymes (NADH and FADH2), and metabolic intermediates for the synthesis of other compounds
-
as carbon dioxide
-
Mitochondria are small organelles with a double membrane that contain the enzymes and other molecules needed for the production of most of the ATP needed by the body.
Key Takeaways
- The acetyl group of acetyl-CoA enters the citric acid cycle. For each acetyl-CoA that enters the citric acid cycle, 2 molecules of carbon dioxide, 3 molecules of NADH, 1 molecule of ATP, and 1 molecule of FADH2 are produced.
- The reduced coenzymes (NADH and FADH2) produced by the citric acid cycle are reoxidized by the reactions of the electron transport chain. This series of reactions also produces a pH gradient across the inner mitochondrial membrane.
- The pH gradient produced by the electron transport chain drives the synthesis of ATP from ADP. For each NADH reoxidized, 2.5–3 molecules of ATP are produced; for each FADH2 reoxidized, 1.5–2 molecules of ATP are produced.
Exercises
-
Replace each question mark with the correct compound.
-
Replace each question mark with the correct compound.
-
From the reactions in Exercises 1 and 2, select the equation(s) by number and letter in which each type of reaction occurs.
- isomerization
- hydration
- synthesis
-
From the reactions in Exercises 1 and 2, select the equation(s) by number and letter in which each type of reaction occurs.
- oxidation
- decarboxylation
- phosphorylation
-
What similar role do coenzyme Q and cytochrome c serve in the electron transport chain?
-
What is the electron acceptor at the end of the electron transport chain? To what product is this compound reduced?
-
What is the function of the cytochromes in the electron transport chain?
-
- What is meant by this statement? “Electron transport is tightly coupled to oxidative phosphorylation.”
- How are electron transport and oxidative phosphorylation coupled or linked?
Answers
-
- citrate
- oxaloacetate + acetyl-CoA
- malate
- α-ketoglutarate hydrogenase complex
-
-
- reaction in 1a
- reaction in 1c
- reaction in 1b
-
-
Both molecules serve as electron shuttles between the complexes of the electron transport chain.
-
-
Cytochromes are proteins in the electron transport chain and serve as one-electron carriers.
-




