This is “End-of-Chapter Material”, section 19.7 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
19.7 End-of-Chapter Material
Chapter Summary
To ensure that you understand the material in this chapter, you should review the meanings of the bold terms in the following summary and ask yourself how they relate to the topics in the chapter.
A cell’s hereditary information is encoded in chromosomes in the cell’s nucleus. Each chromosome is composed of proteins and deoxyribonucleic acid (DNA). The chromosomes contain smaller hereditary units called genes, which are relatively short segments of DNA. The hereditary information is expressed or used through the synthesis of ribonucleic acid (RNA). Both nucleic acids—DNA and RNA—are polymers composed of monomers known as nucleotides, which in turn consist of phosphoric acid (H3PO4), a nitrogenous base, and a pentose sugar.
The two types of nitrogenous bases most important in nucleic acids are purines—adenine (A) and guanine (G)—and pyrimidines—cytosine (C), thymine (T), and uracil (U). DNA contains the nitrogenous bases adenine, cytosine, guanine, and thymine, while the bases in RNA are adenine, cytosine, guanine, and uracil. The sugar in the nucleotides of RNA is ribose; the one in DNA is 2-deoxyribose. The sequence of nucleotides in a nucleic acid defines the primary structure of the molecule.
RNA is a single-chain nucleic acid, whereas DNA possesses two nucleic-acid chains intertwined in a secondary structure called a double helix. The sugar-phosphate backbone forms the outside the double helix, with the purine and pyrimidine bases tucked inside. Hydrogen bonding between complementary bases holds the two strands of the double helix together; A always pairs with T and C always pairs with G.
Cell growth requires replication, or reproduction of the cell’s DNA. The double helix unwinds, and hydrogen bonding between complementary bases breaks so that there are two single strands of DNA, and each strand is a template for the synthesis of a new strand. For protein synthesis, three types of RNA are needed: messenger RNA (mRNA), ribosomal RNA (rRNA), and transfer RNA (tRNA). All are made from a DNA template by a process called transcription. The double helix uncoils, and ribonucleotides base-pair to the deoxyribonucleotides on one DNA strand; however, RNA is produced using uracil rather than thymine. Once the RNA is formed, it dissociates from the template and leaves the nucleus, and the DNA double helix reforms.
Translation is the process in which proteins are synthesized from the information in mRNA. It occurs at structures called ribosomes, which are located outside the nucleus and are composed of rRNA and protein. The 64 possible three-nucleotide combinations of the 4 nucleotides of DNA constitute the genetic code that dictates the sequence in which amino acids are joined to make proteins. Each three-nucleotide sequence on mRNA is a codon. Each kind of tRNA molecule binds a specific amino acid and has a site containing a three-nucleotide sequence called an anticodon.
The general term for any change in the genetic code in an organism’s DNA is mutation. A change in which a single base is substituted, inserted, or deleted is a point mutation. The chemical and/or physical agents that cause mutations are called mutagens. Diseases that occur due to mutations in critical DNA sequences are referred to as genetic diseases.
Viruses are infectious agents composed of a tightly packed central core of nucleic acids enclosed by a protective shell of proteins. Viruses contain either DNA or RNA as their genetic material but not both. Some RNA viruses, called retroviruses, synthesize DNA in the host cell from their RNA genome. The human immunodeficiency virus (HIV) causes acquired immunodeficiency syndrome (AIDS).
Additional Exercises
-
For this nucleic acid segment,
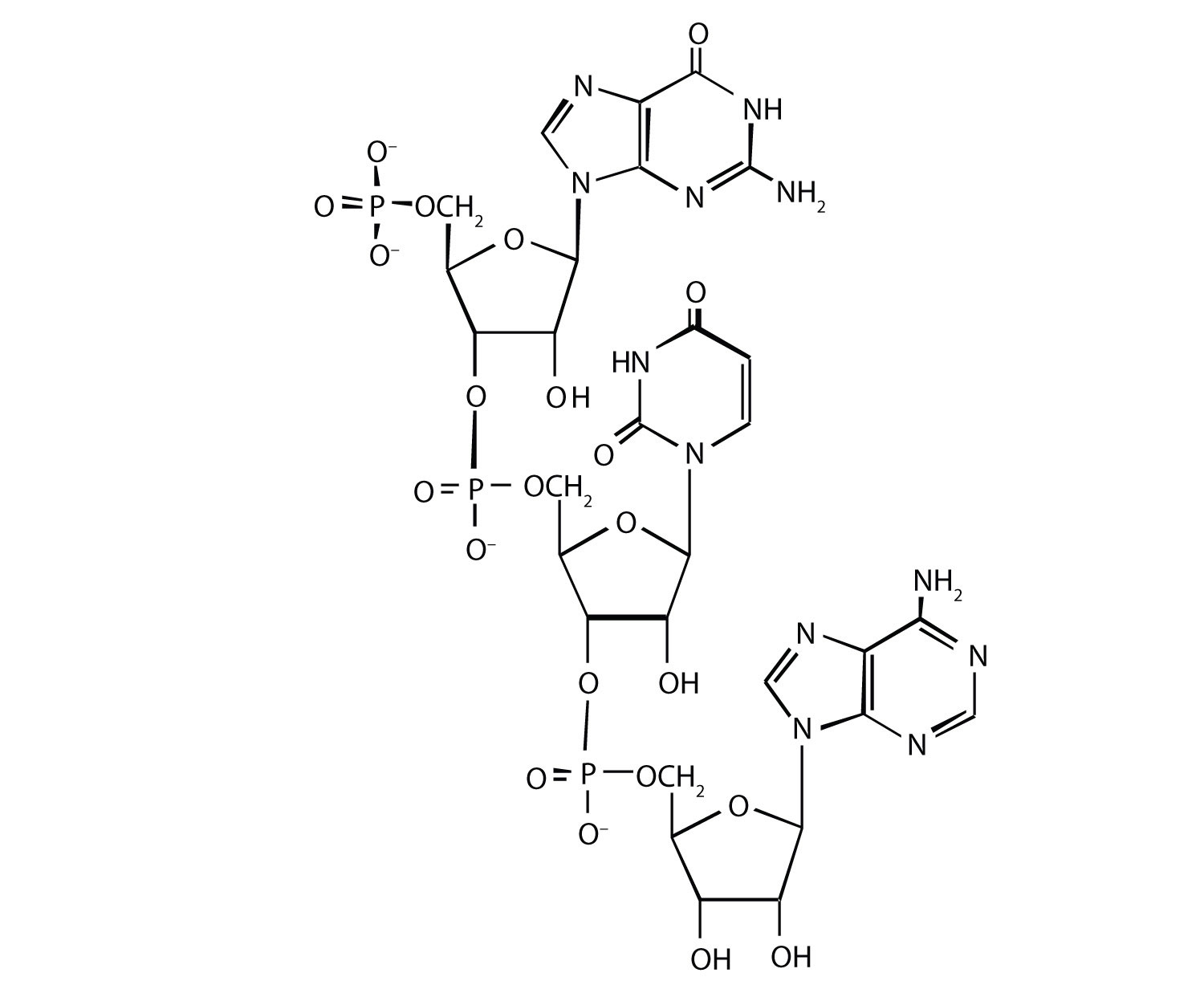
- classify this segment as RNA or DNA and justify your choice.
- determine the sequence of this segment, labeling the 5′ and 3′ ends.
-
For this nucleic acid segment,
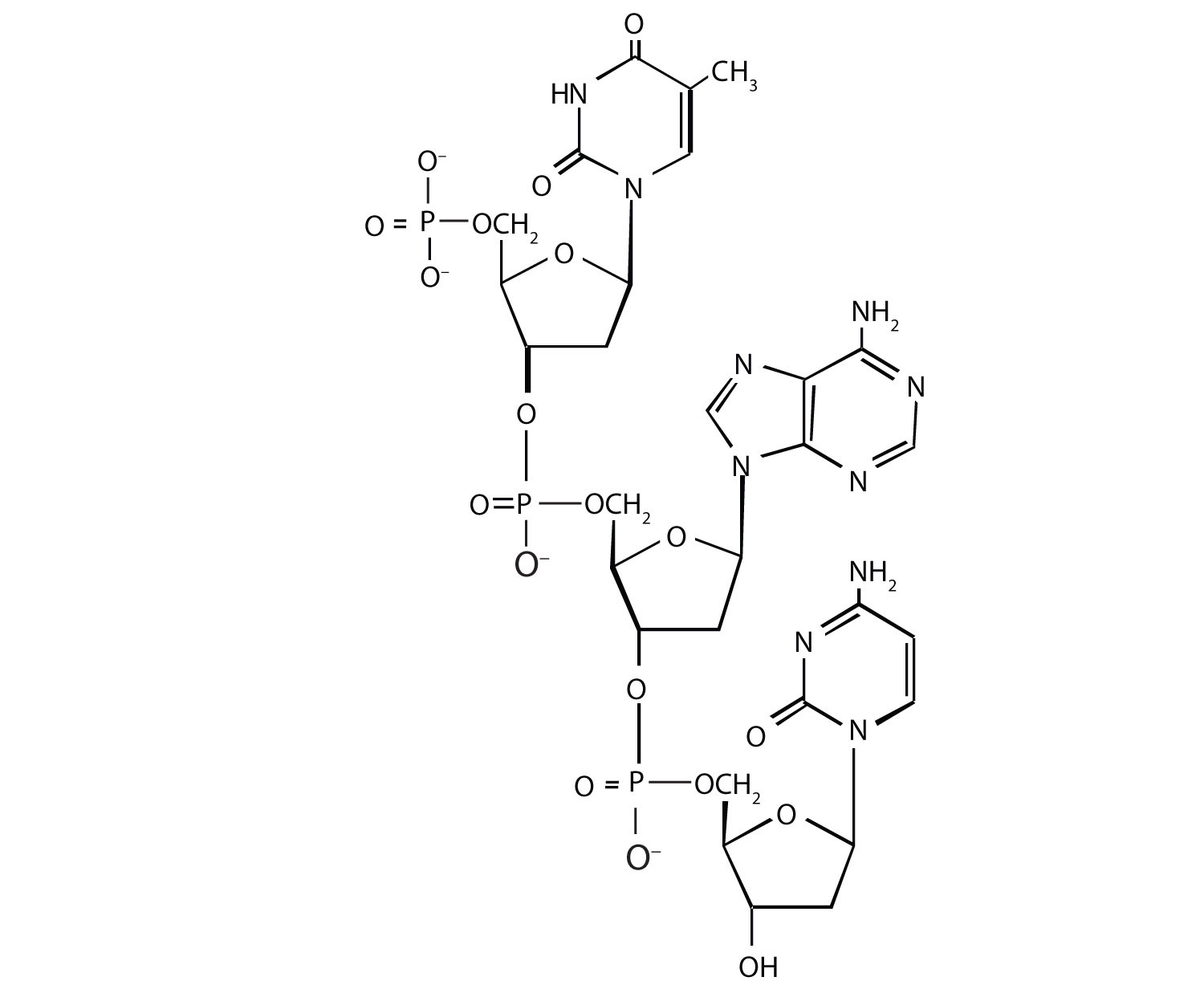
- classify this segment as RNA or DNA and justify your choice.
- determine the sequence of this segment, labeling the 5′ and 3′ ends.
-
One of the key pieces of information that Watson and Crick used in determining the secondary structure of DNA came from experiments done by E. Chargaff, in which he studied the nucleotide composition of DNA from many different species. Chargaff noted that the molar quantity of A was always approximately equal to the molar quantity of T, and the molar quantity of C was always approximately equal to the molar quantity of G. How were Chargaff’s results explained by the structural model of DNA proposed by Watson and Crick?
-
Suppose Chargaff (see Exercise 3) had used RNA instead of DNA. Would his results have been the same; that is, would the molar quantity of A approximately equal the molar quantity of T? Explain.
-
In the DNA segment
5′‑ATGAGGCATGAGACG‑3′ (coding strand) 3′‑TACTCCGTACTCTGC‑5′ (template strand)- What products would be formed from the segment’s replication?
- Write the mRNA sequence that would be obtained from the segment’s transcription.
- What is the amino acid sequence of the peptide produced from the mRNA in Exercise 5b?
-
In the DNA segment
5′‑ATGACGGTTTACTAAGCC‑3′ (coding strand) 3′‑TACTGCCAAATGATTCGG‑5′ (template strand)- What products would be formed from the segment’s replication?
- Write the mRNA sequence that would be obtained from the segment’s transcription.
- What is the amino acid sequence of the peptide produced from the mRNA in Exercise 6b?
-
A hypothetical protein has a molar mass of 23,300 Da. Assume that the average molar mass of an amino acid is 120.
- How many amino acids are present in this hypothetical protein?
- What is the minimum number of codons present in the mRNA that codes for this protein?
- What is the minimum number of nucleotides needed to code for this protein?
-
Bradykinin is a potent peptide hormone composed of nine amino acids that lowers blood pressure.
- The amino acid sequence for bradykinin is arg-pro-pro-gly-phe-ser-pro-phe-arg. Postulate a base sequence in the mRNA that would direct the synthesis of this hormone. Include an initiation codon and a termination codon.
- What is the nucleotide sequence of the DNA that codes for this mRNA?
-
A particular DNA coding segment is ACGTTAGCCCCAGCT.
- Write the sequence of nucleotides in the corresponding mRNA.
- Determine the amino acid sequence formed from the mRNA in Exercise 9a during translation.
-
What amino acid sequence results from each of the following mutations?
- replacement of the underlined guanine by adenine
- insertion of thymine immediately after the underlined guanine
- deletion of the underlined guanine
-
A particular DNA coding segment is TACGACGTAACAAGC.
- Write the sequence of nucleotides in the corresponding mRNA.
- Determine the amino acid sequence formed from the mRNA in Exercise 10a during translation.
-
What amino acid sequence results from each of the following mutations?
- replacement of the underlined guanine by adenine
- replacement of the underlined adenine by thymine
-
Two possible point mutations are the substitution of lysine for leucine or the substitution of serine for threonine. Which is likely to be more serious and why?
-
Two possible point mutations are the substitution of valine for leucine or the substitution of glutamic acid for histidine. Which is likely to be more serious and why?
Answers
-
- RNA; the sugar is ribose, rather than deoxyribose
- 5′‑GUA‑3′
-
-
In the DNA structure, because guanine (G) is always paired with cytosine (C) and adenine (A) is always paired with thymine (T), you would expect to have equal amounts of each.
-
-
- Each strand would be replicated, resulting in two double-stranded segments.
- 5′‑AUGAGGCAUGAGACG‑3′
- met-arg-his-glu-thr
-
-
- 194
- 194
- 582
-
-
- 5′‑ACGUUAGCCCCAGCU‑3′
- thr-leu-ala-pro-ala
-
- thr-leu-thr-pro-ala
- thr-leu-val-pro-ser
- thr-leu-pro-gin
-
-
substitution of lysine for leucine because you are changing from an amino acid with a nonpolar side chain to one that has a positively charged side chain; both serine and threonine, on the other hand, have polar side chains containing the OH group.
-




