This is “End-of-Chapter Material”, section 16.8 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
16.8 End-of-Chapter Material
Chapter Summary
To ensure that you understand the material in this chapter, you should review the meanings of the bold terms in the following summary and ask yourself how they relate to the topics in the chapter.
Carbohydrates, a large group of biological compounds containing carbon, hydrogen, and oxygen atoms, include sugars, starch, glycogen, and cellulose. All carbohydrates contain alcohol functional groups, and either an aldehyde or a ketone group (or a functional group that can be converted to an aldehyde or ketone). The simplest carbohydrates are monosaccharides. Those with two monosaccharide units are disaccharides, and those with many monosaccharide units are polysaccharides. Most sugars are either monosaccharides or disaccharides. Cellulose, glycogen, and starch are polysaccharides.
Many carbohydrates exist as stereoisomers, in which the three-dimensional spatial arrangement of the atoms in space is the only difference between the isomers. These particular stereoisomers contain at least one chiral carbon, a carbon atom that has four different groups bonded to it. A molecule containing a chiral carbon is nonsuperimposable on its mirror image, and two molecules that are nonsuperimposable mirror images of each other are a special type of stereoisomer called enantiomers. Enantiomers have the same physical properties, such as melting point, but differ in the direction they rotate polarized light.
A sugar is designated as being a D sugar or an L sugar according to how, in a Fischer projection of the molecule, the hydrogen atom and OH group are attached to the penultimate carbon atom, which is the carbon atom immediately before the terminal alcohol carbon atom. If the structure at this carbon atom is the same as that of D-glyceraldehyde (OH to the right), the sugar is a D sugar; if the configuration is the same as that of L-glyceraldehyde (OH to the left), the sugar is an L sugar.
Monosaccharides of five or more carbons atoms readily form cyclic structures when the carbonyl carbon atom reacts with an OH group on a carbon atom three or four carbon atoms distant. Consequently, glucose in solution exists as an equilibrium mixture of three forms, two of them cyclic (α- and β-) and one open chain. In Haworth projections, the alpha form is drawn with the OH group on the “former” carbonyl carbon atom (anomeric carbon) pointing downward; the beta form, with the OH group pointing upward; these two compounds are stereoisomers and are given the more specific term of anomers. Any solid sugar can be all alpha or all beta. Once the sample is dissolved in water, however, the ring opens up into the open-chain structure and then closes to form either the α- or the β-anomer. These interconversions occur back and forth until a dynamic equilibrium mixture is achieved in a process called mutarotation.
The carbonyl group present in monosaccharides is easily oxidized by Tollens’ or Benedict’s reagents (as well as others). Any mono- or disaccharide containing a free anomeric carbon is a reducing sugar. The disaccharide maltose contains two glucose units joined in an α-1,4-glycosidic linkage. The disaccharide lactose contains a galactose unit and a glucose unit joined by a β-1,4-glycosidic linkage. Both maltose and lactose contain a free anomeric carbon that can convert to an aldehyde functional group, so they are reducing sugars; they also undergo mutarotation. Many adults, and some children, have a deficiency of the enzyme lactase (which is needed to break down lactose) and are said to be lactose intolerant. A more serious problem is the genetic disease galactosemia, which results from the absence of an enzyme needed to convert galactose to glucose.
The disaccharide sucrose (table sugar) consists of a glucose unit and a fructose unit joined by a glycosidic linkage. The linkage is designated as an α-1,β-2-glycosidic linkage because it involves the OH group on the first carbon atom of glucose and the OH group on the second carbon atom of fructose. Sucrose is not a reducing sugar because it has no anomeric carbon that can reform a carbonyl group, and it cannot undergo mutarotation because of the restrictions imposed by this linkage.
Starch, the principal carbohydrate of plants, is composed of the polysaccharides amylose (10%–30%) and amylopectin (70%–90%). When ingested by humans and other animals, starch is hydrolyzed to glucose and becomes the body’s energy source. Glycogen is the polysaccharide animals use to store excess carbohydrates from their diets. Similar in structure to amylopectin, glycogen is hydrolyzed to glucose whenever an animal needs energy for a metabolic process. The polysaccharide cellulose provides structure for plant cells. It is a linear polymer of glucose units joined by β-1,4-glycosidic linkages. It is indigestible in the human body but digestible by many microorganisms, including microorganisms found in the digestive tracts of many herbivores.
Additional Exercises
-
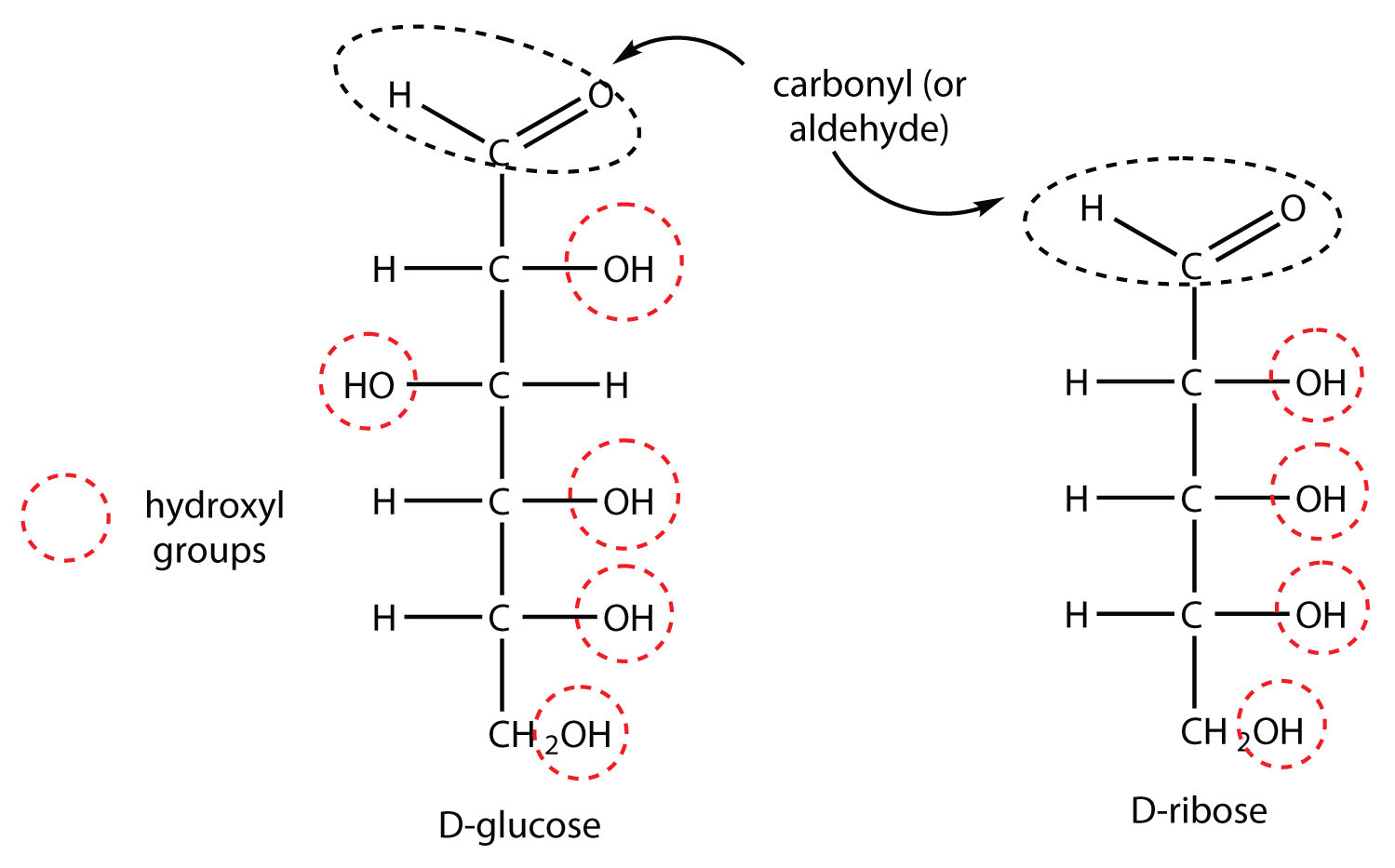
Draw the Fischer projections for D-glucose and D-ribose. Identify all the functional groups in each structure.
-
Draw the Fischer projections for D-galactose and D-fructose. Identify all the functional groups in each structure.
-
L-Fucose is an aldohexose that is often incorporated into oligosaccharides attached to cell membranes. It is also known as 6-deoxy-L-galactose. Draw the structure of L-fucose.
-
D-glucitol, also known as sorbitol, is added to shredded coconut to keep it soft and to pharmaceutical products to increase the absorption of nutrients. It is prepared industrially by the reduction of D-glucose. Propose a structure for D-glucitol.
-
Which would give a positive Benedict’s test—lactose, amylopectin, D-ribose, sucrose, D-glyceraldehyde, or amylose?
-
Which enzyme hydrolyzes each carbohydrate?
- maltose
- lactose
- cellulose
- sucrose
-
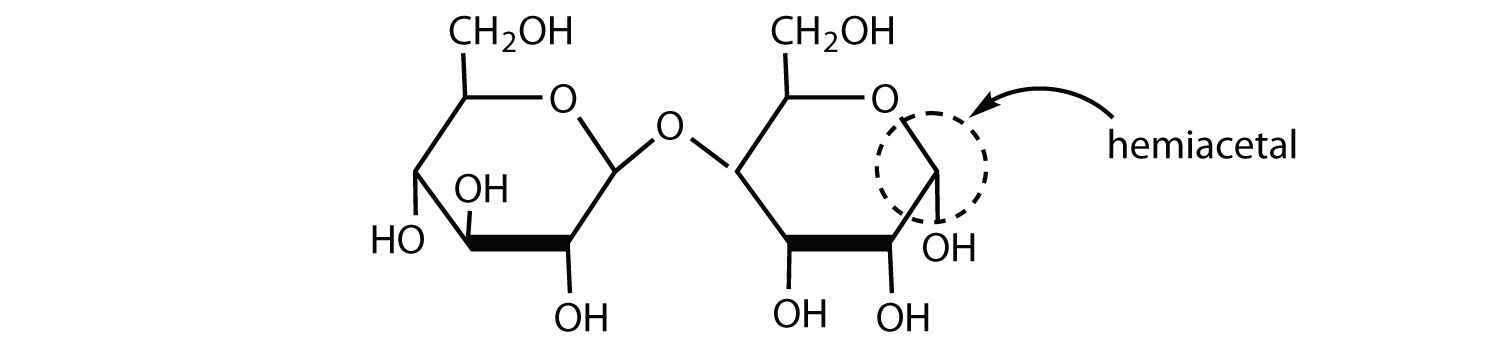
What structural characteristics are necessary if a disaccharide is to be a reducing sugar? Draw the structure of a hypothetical reducing disaccharide composed of two aldohexoses.
-
Raffinose, a trisaccharide found in beans and sugar beets, contains D-galactose, D-glucose, and D-fructose. The enzyme α-galactase catalyzes the hydrolysis of raffinose to galactose and sucrose. Draw the structure of raffinose. (The linkage from galactose to the glucose unit is α-1,6).
-
What reagent(s) could be used to carry out each conversion?
-
-
What reagents are necessary to carry out each conversion?
-
-
The structure of lactulose is shown here. What monosaccharide units compose this disaccharide?
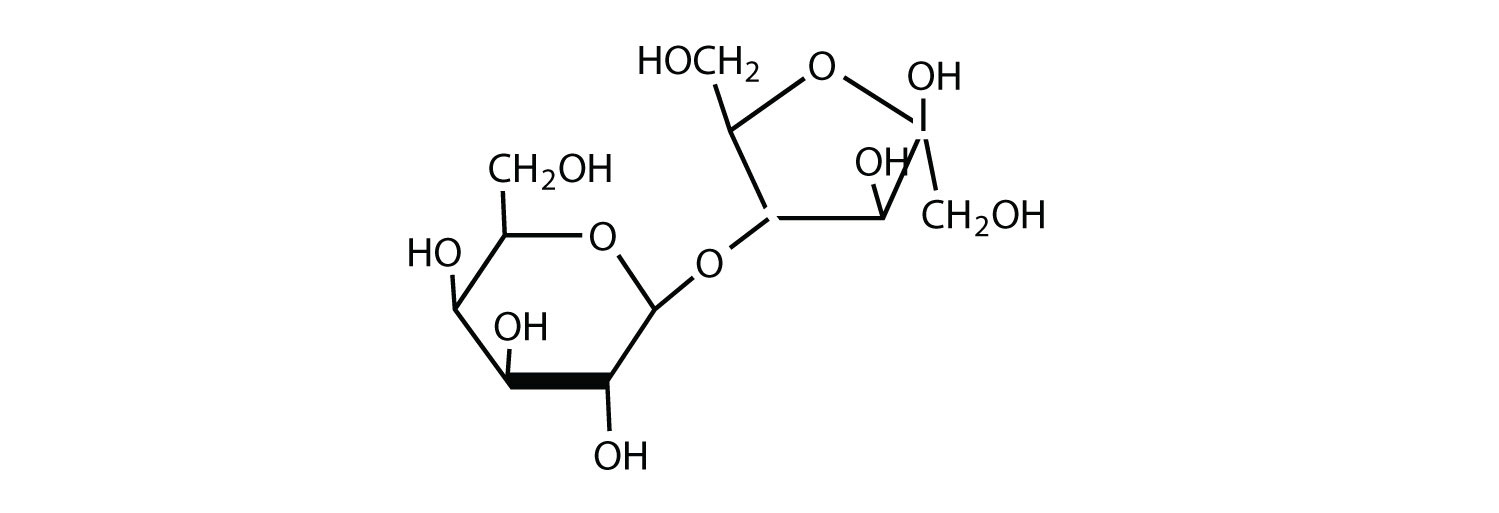
-
N-acetylglucosamine is synthesized from D-glucosamine, which in turn is obtained from D-glucose. What reagents are needed for the conversion of D-glucosamine to N-acetylglucosamine?
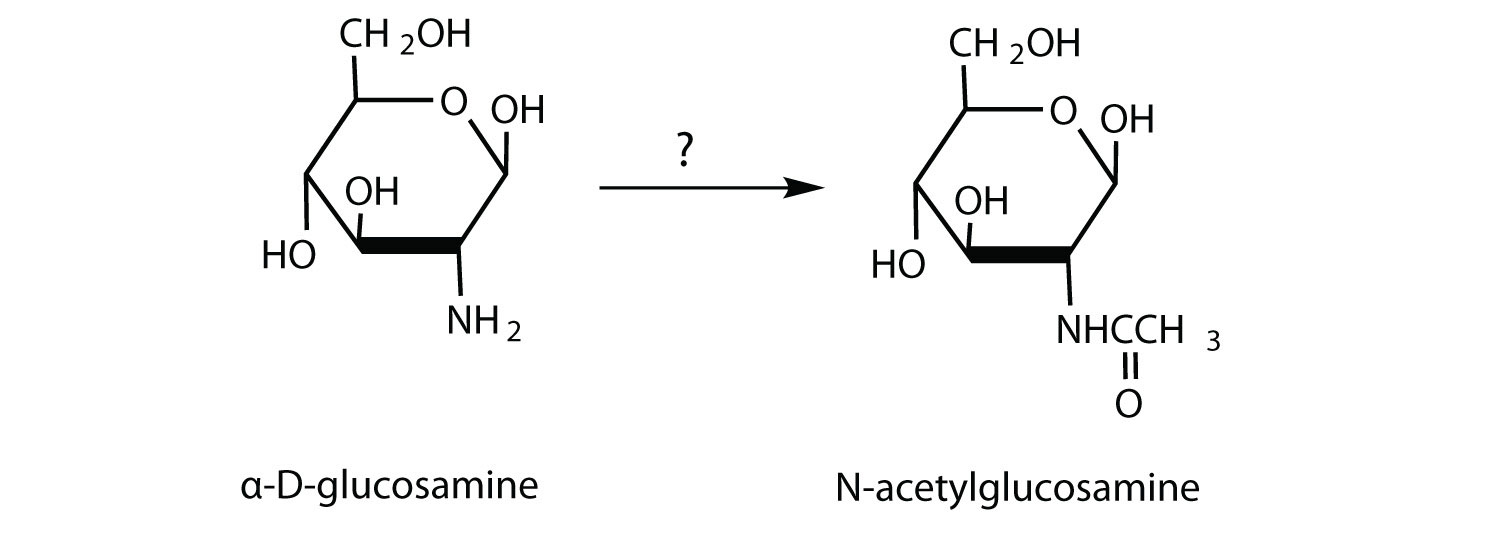
-
Hyaluronic acid is a heteropolymer that acts as a lubricating agent in the fluids of joints and the eyes. Its structure consists of repeating disaccharide units containing glucuronic acid and N-acetylglucosamine connected by a β-1,3-linkage. Draw the structure of the disaccharide unit found in hyaluronic acid.
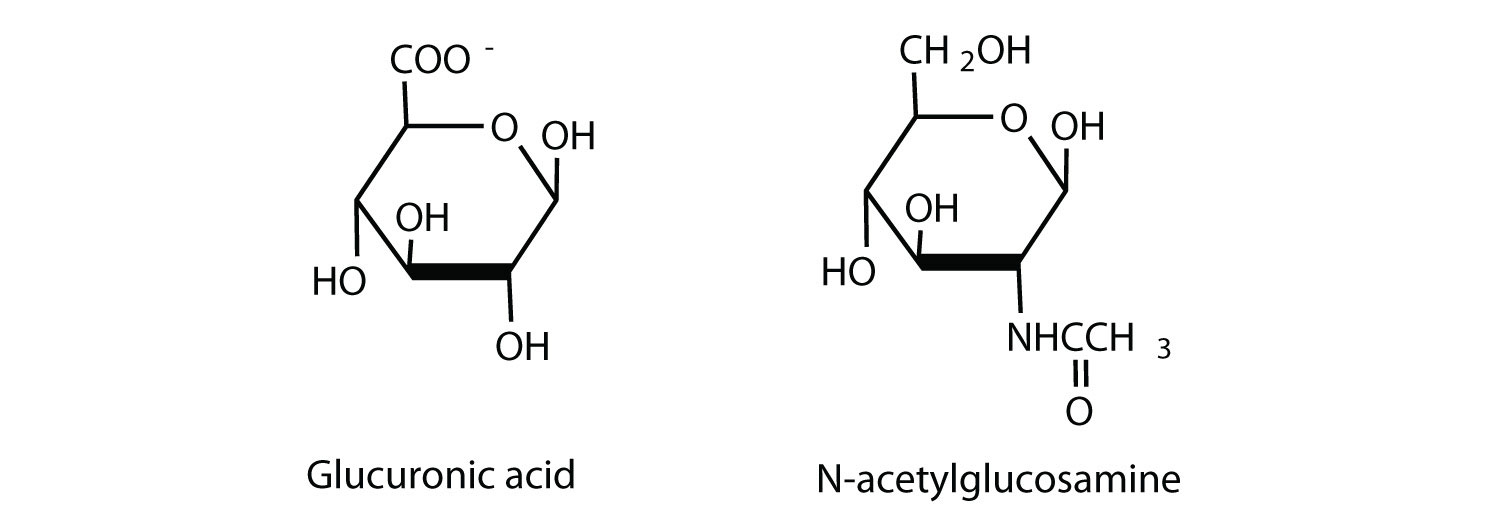
-
Several artificial sweeteners are discussed in this chapter.
- Which are currently approved for use in the United States?
- Which has (or have) a bitter, metallic aftertaste?
- Which was (or were) most recently approved for use in the United States?
- Which contain(s) potassium?
-
If 3.0 mmol (3.0 × 10−3 mol) samples of saccharin, cyclamate, aspartame, and acesulfame K were each dissolved in separate beakers containing 500 mL of pure water, which solution would have the sweetest taste? Which solution would have the least sweet taste? Justify your answers.
-
Identify two functional groups found in aspartame, acesulfame K, and sucralose.
-
Why does a deficiency of lactase lead to cramps and diarrhea?
-
How does galactosemia differ from lactose intolerance in terms of the cause of the disease and its symptoms and severity?
Answers
-
-
-
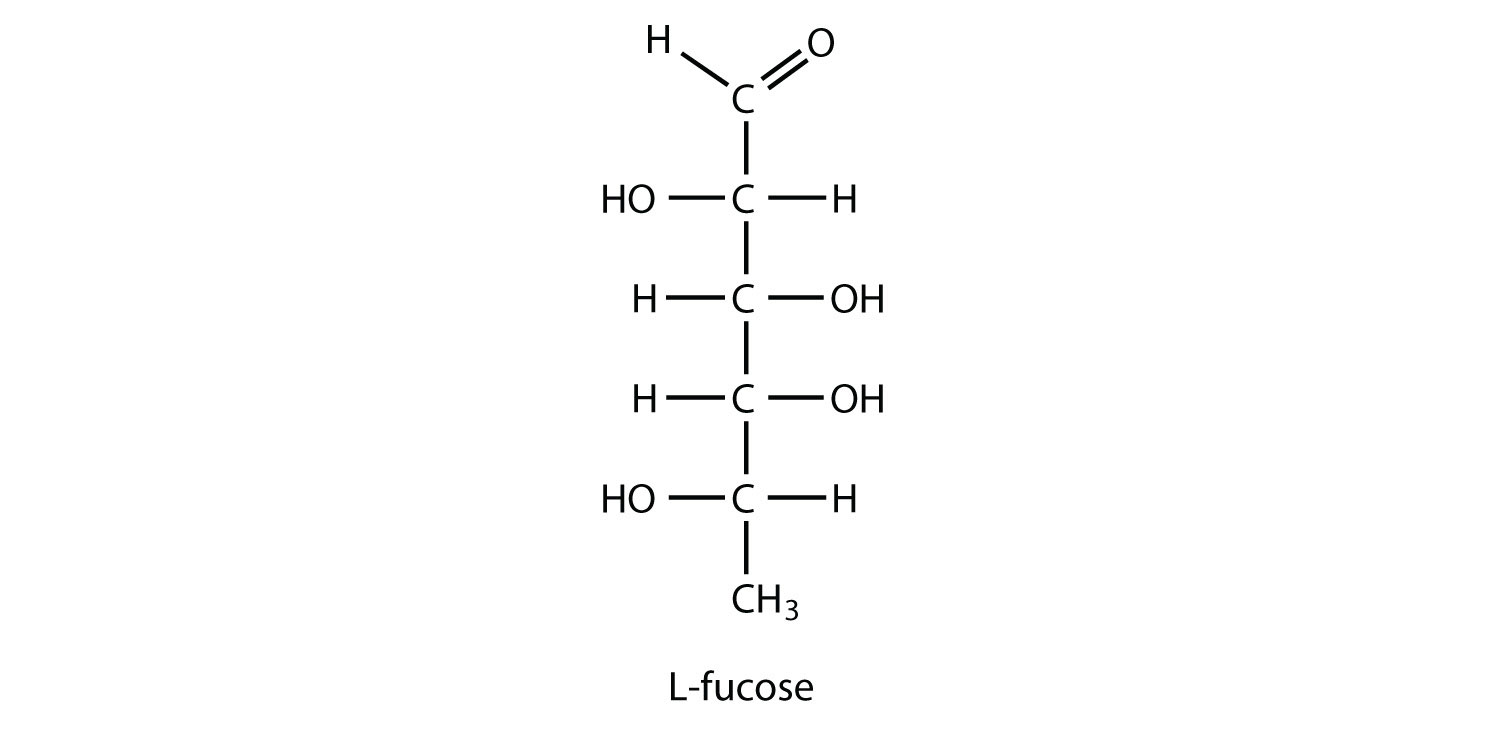
-
-
Lactose, D-ribose, and D-glyceraldehyde would give a positive Benedict’s test.
-
-
To be a reducing sugar, a disaccharide must contain an anomeric carbon atom that can open up to form an aldehyde functional group, as shown in this disaccharide (answers will vary).
-
-
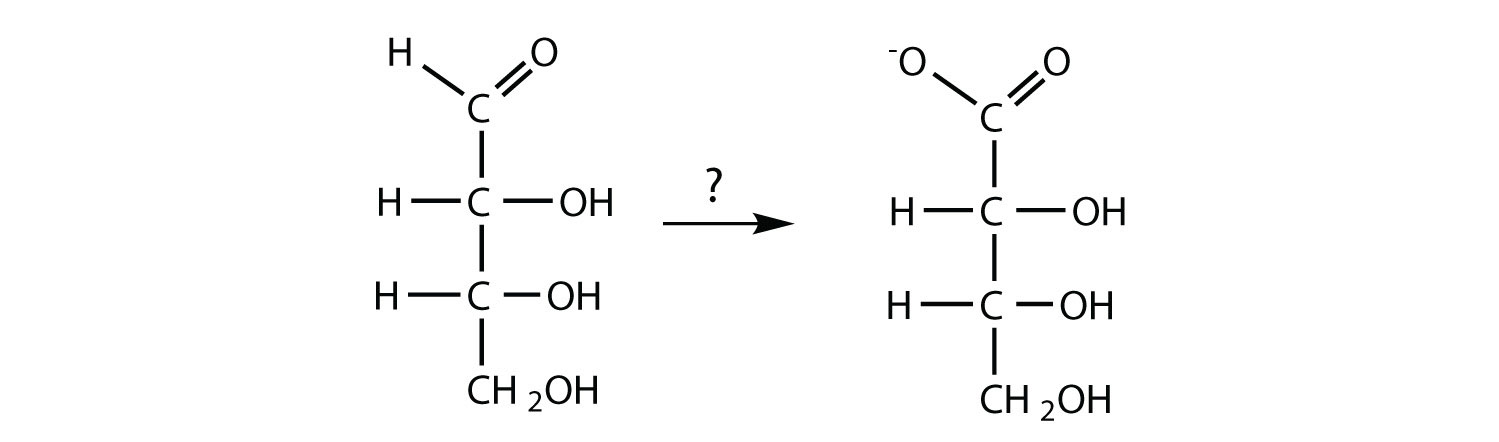
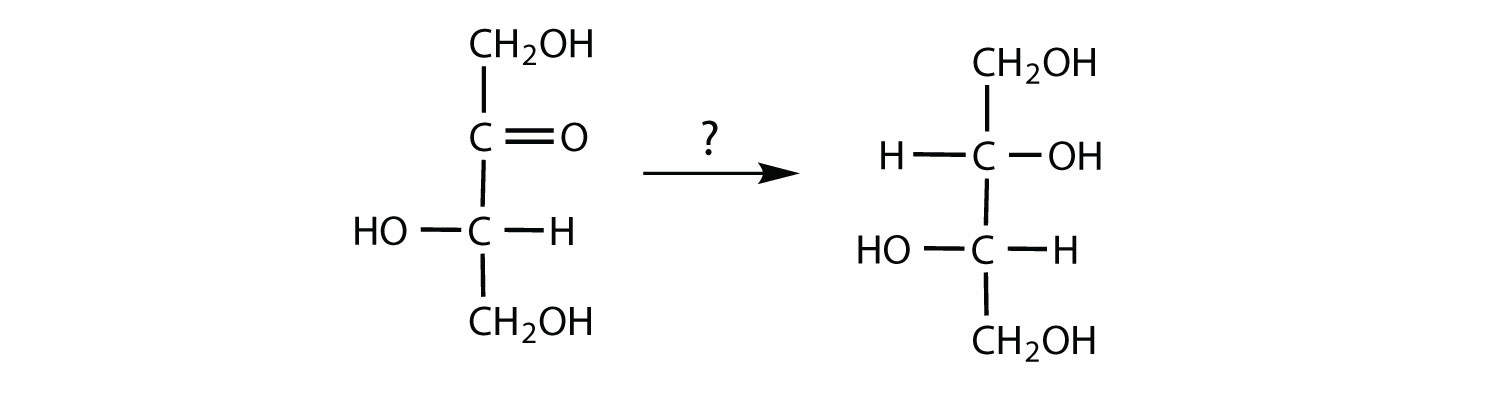
- The carbohydrate is being oxidized; Tollens’ or Benedict’s reagent could be used.
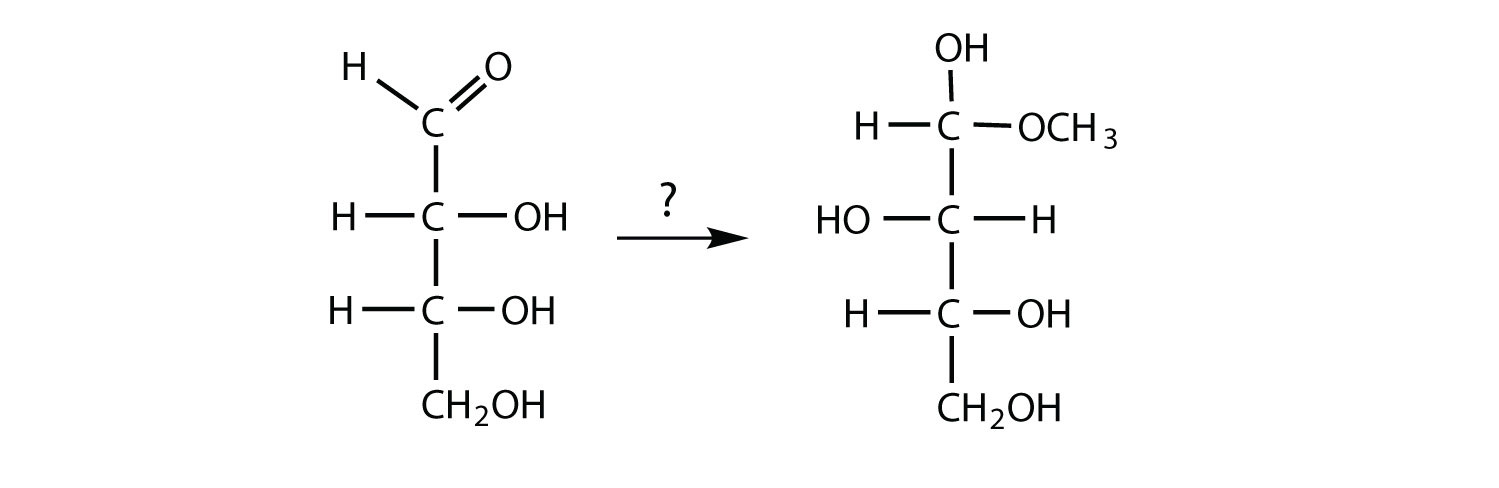
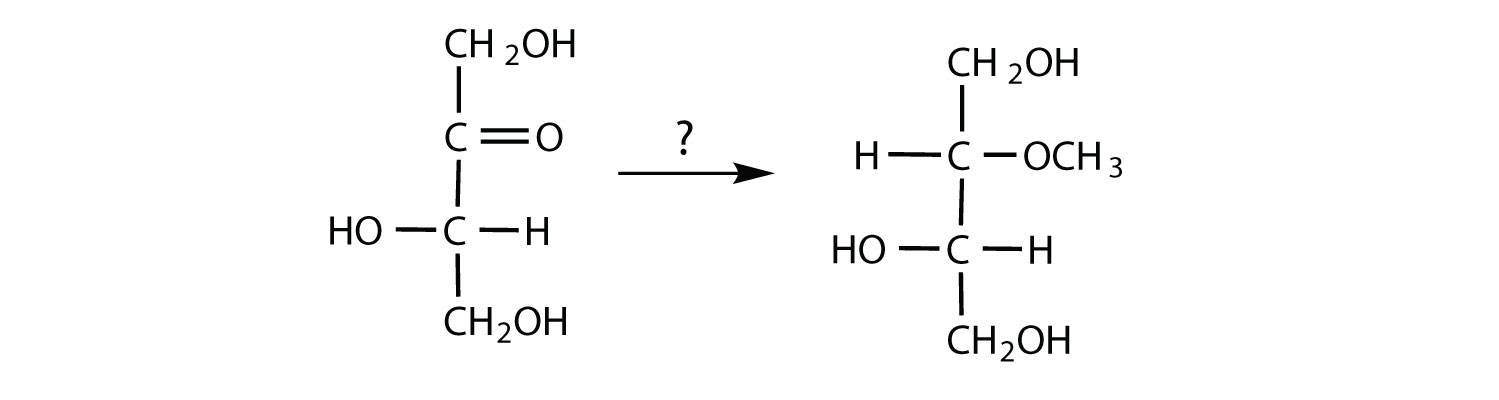
- To form the compound shown, an aldehyde must react with methanol (CH3OH) and an acid catalyst.
-
-
galactose and fructose
-
-
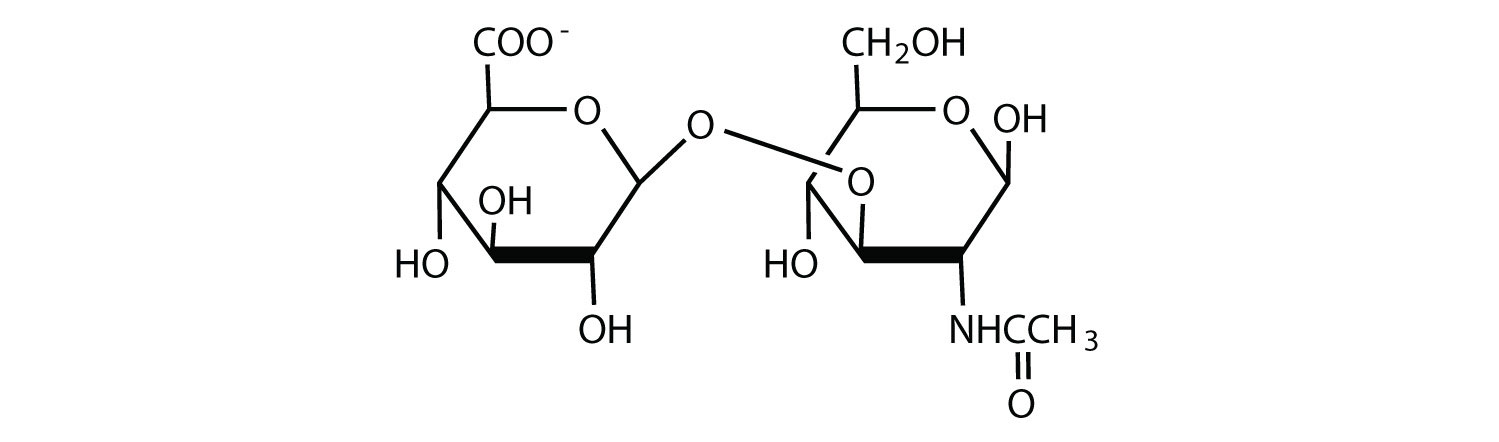
-
-
Sucralose would be expected to have the sweetest taste because its relative sweetness is the highest. Lactose would have the least sweet taste because it has the lowest relative sweetness.
-
-
Intestinal bacteria can act on the lactose present in the intestine to produce organic acids and gases. The buildup of water and bacterial decay products leads to cramps and diarrhea.
-