This is “End-of-Chapter Material”, section 15.18 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
15.18 End-of-Chapter Material
Chapter Summary
To ensure that you understand the material in this chapter, you should review the meanings of the following bold terms in the summary and ask yourself how they relate to the topics in the chapter.
A carboxylic acid (RCOOH) contains the functional group COOH, called the carboxyl group, which has an OH group attached to a carbonyl carbon atom. An ester (RCOOR′) has an OR′ group attached to a carbonyl carbon atom. An amine is derived from ammonia (NH3), with one, two, or all three of the hydrogen atoms of NH3 replaced by an alkyl (or an aryl) group. The amide functional group has a carbonyl group joined to a nitrogen atom from NH3 or an amine.
There are many familiar carboxylic acids. The R group may be a hydrogen atom (as in formic acid, HCOOH), an alkyl group (as in acetic acid, CH2COOH), or an aryl group (as in benzoic acid, C6H5COOH). The location of substituents along the carbon chain is indicated by a Greek letter (for common names) or a number (for names from the International Union of Pure and Applied Chemistry).
A carboxylic acid is formed by the oxidation of an aldehyde with the same number of carbon atoms. Because aldehydes are formed from primary alcohols, these alcohols are also a starting material for carboxylic acids.
Carboxylic acids have strong, often disagreeable, odors. They are highly polar molecules and readily engage in hydrogen bonding, so they have relatively high boiling points.
Carboxylic acids are weak acids. They react with bases to form salts and with carbonates and bicarbonates to form carbon dioxide gas and the salt of the acid.
Esters are pleasant-smelling compounds that are responsible for the fragrances of flowers and fruits. They have lower boiling points than comparable carboxylic acids because, even though ester molecules are somewhat polar, they cannot engage in hydrogen bonding. However, with water, esters can engage in hydrogen bonding; consequently, the low molar mass esters are soluble in water. Esters can be synthesized by esterification, in which a carboxylic acid and an alcohol are combined under acidic conditions. Esters are neutral compounds that undergo hydrolysis, a reaction with water. Under acidic conditions, hydrolysis is essentially the reverse of esterification. When carried out under basic conditions, the process is called saponification.
Inorganic acids also react with alcohols to form esters. Some of the most important esters in biochemistry are those formed from phosphoric acid.
Amines are nitrogen-containing organic molecules derived from ammonia (NH3). A primary (1°) amine (RNH2) has one organic group bonded to the nitrogen atom, a secondary (2°) amine (R2NH) has two organic groups bonded to the nitrogen atom, and a tertiary (3°) amine (R3N) has three organic groups bonded to the nitrogen atom. Amines are basic compounds that react with strong acids to produce ammonium (NH4+) salts. A cyclic compound in which the ring contains one or more noncarbon atoms is called a heterocyclic compound. There are many heterocyclic amines, including many physiologically important ones. Alkaloids are heterocyclic amines found in many plants. Caffeine, nicotine, and cocaine are familiar alkaloids.
Organic compounds containing a carbonyl group bonded to a nitrogen atom are amides, and the carbon-to-nitrogen bond is an amide linkage (or a peptide linkage). Most amides are colorless and odorless, and the lighter ones are soluble in water. Because they are polar molecules, amides have comparatively high boiling points and melting points. Amides are synthesized from carboxylic acids and NH3 or amines. Amides are neutral compounds. They resist hydrolysis in water, but acids, bases, and enzymes catalyze the reaction.
Additional Exercises
-
Of the families of organic compounds discussed in this chapter, which are known for their typically unpleasant odors? Which for their characteristically pleasant aromas?
-
What is esterification of a carboxylic acid? How does it differ from neutralization?
-
Like alcohols, phenols form esters with carboxylic acids. The hydrocarbon group from phenol is called phenyl. Draw the structure of phenyl acetate.
-
Describe the hydrogen bonding in carboxylic acids, both acid-acid and acid-water. How does this influence their physical properties?
-
Which compound is more soluble in water—benzoic acid or sodium benzoate? Explain.
-
Dicarboxylic acids have two carboxyl groups and are named with the ending -dioic acid. Give the equation for the reaction of 1,5-pentanedioic acid (HOOCCH2CH2CH2COOH; common name, glutaric acid) with each of the following:
- 1 mol of NaOH
- 2 mol of NaOH
-
Without consulting tables, arrange the following compounds in order of increasing boiling point: butyl alcohol, methyl acetate, pentane, and propionic acid.
-
From which alcohol might each acid be prepared via oxidation with acidic dichromate?
- CH3CH2COOH
- HCOOH
- HOOCH2COOH
- (CH3)2CHCH2COOH
-
The distinctive aroma and flavor of oranges are due in part to octyl acetate, an ester formed from 1-octanol (octyl alcohol) and acetic acid. Write the condensed structural formula for octyl acetate.
-
A lactone is a cyclic ester. What product is formed in following reaction?
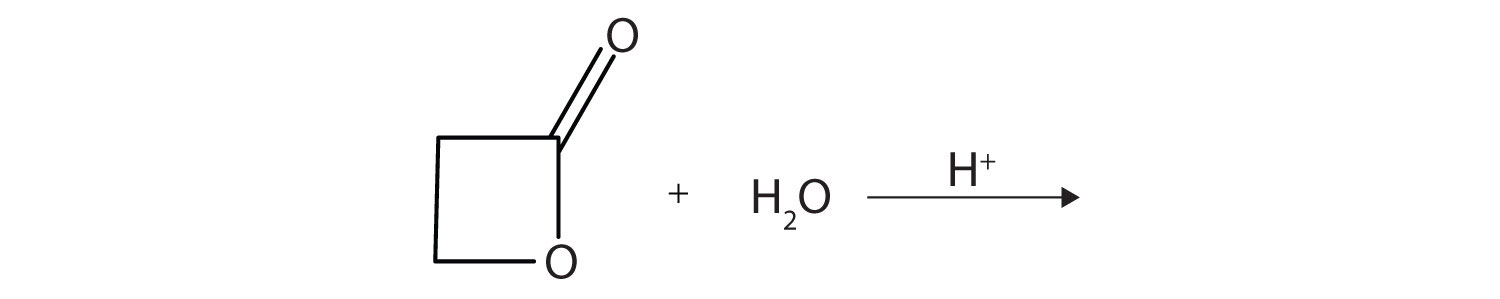
-
A lactam is a cyclic amide. What product is formed in the following reaction?
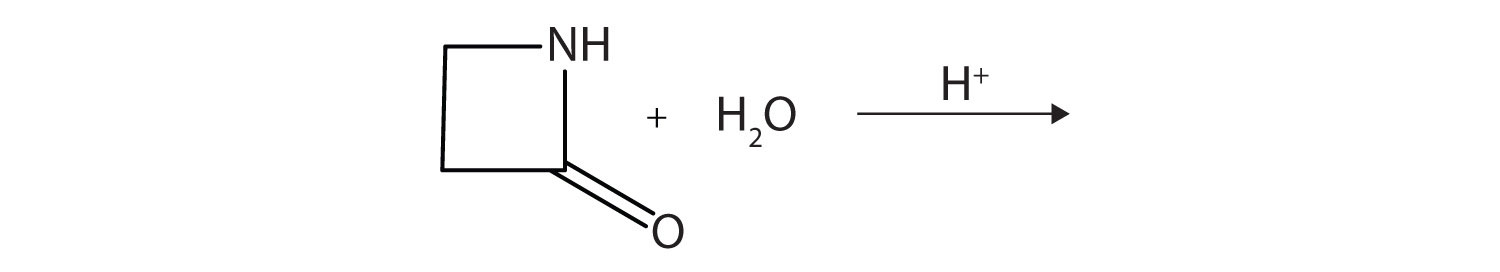
-
Draw the structures for the eight isomeric amines that have the molecular formula C4H11N. Give each a common name and classify it as primary, secondary, or tertiary.
-
Draw the structures for the five isomeric amines that have the molecular formula C7H9N and contain a benzene ring. Classify each compound as primary, secondary, or tertiary.
-
Cocaine is usually used in the form of the salt cocaine hydrochloride and sniffed up the nose. Some prefer to ingest their cocaine by smoking it (mixed with tobacco, for example). Before smoking, the cocaine hydrochloride must be converted back to the free base (that is, to the molecular form). Explain the choice of dosage form for each route of administration.
-
Draw the structures all the isomeric amides that have the molecular formula C4H9NO.
-
An ester with the molecular formula C6H12O2 was hydrolyzed in aqueous acid to yield an acid Y and an alcohol Z. Oxidation of the alcohol with potassium dichromate (K2Cr2O7) gave the identical acid Y. What is the condensed structural formula of the ester?
-
The neutralization of 125 mL of a 0.400 M NaOH solution requires 5.10 g of a monocarboxylic acid. Draw all the possible structures for the acid.
-
If 3.00 g of acetic acid reacts with excess methanol, how many grams of methyl acetate are formed?
-
How many milliliters of a 0.100 M barium hydroxide solution are required to neutralize 0.500 g of dichloroacetic acid?
Answers
-
unpleasant: carboxylic acids; pleasant: esters
-
-
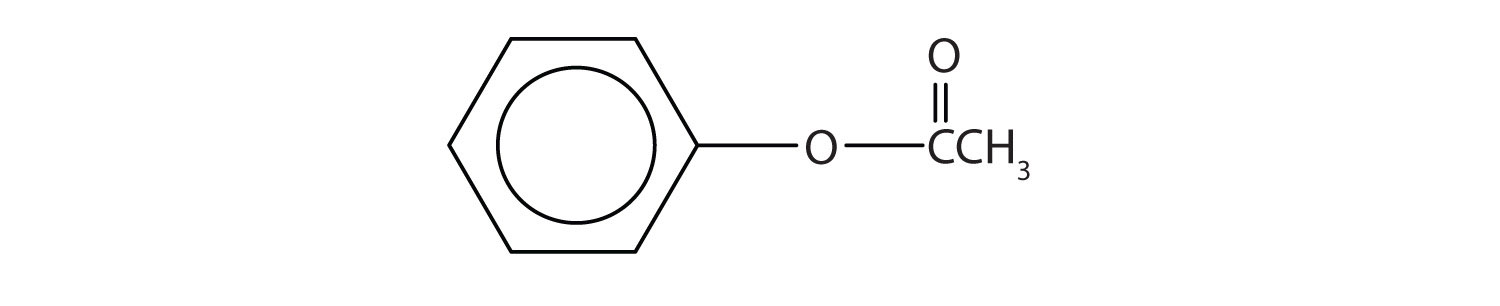
-
-
sodium benzoate because it is ionic and forms ion-dipole forces with water; benzoic acid can engage in hydrogen bonding only with water
-
-
pentane < methyl acetate < butyl alcohol < propionic acid
-
-
CH3COOCH2CH2CH2CH2CH2CH2CH2CH3
-
-
H3N+CH2CH2COOH
-
-
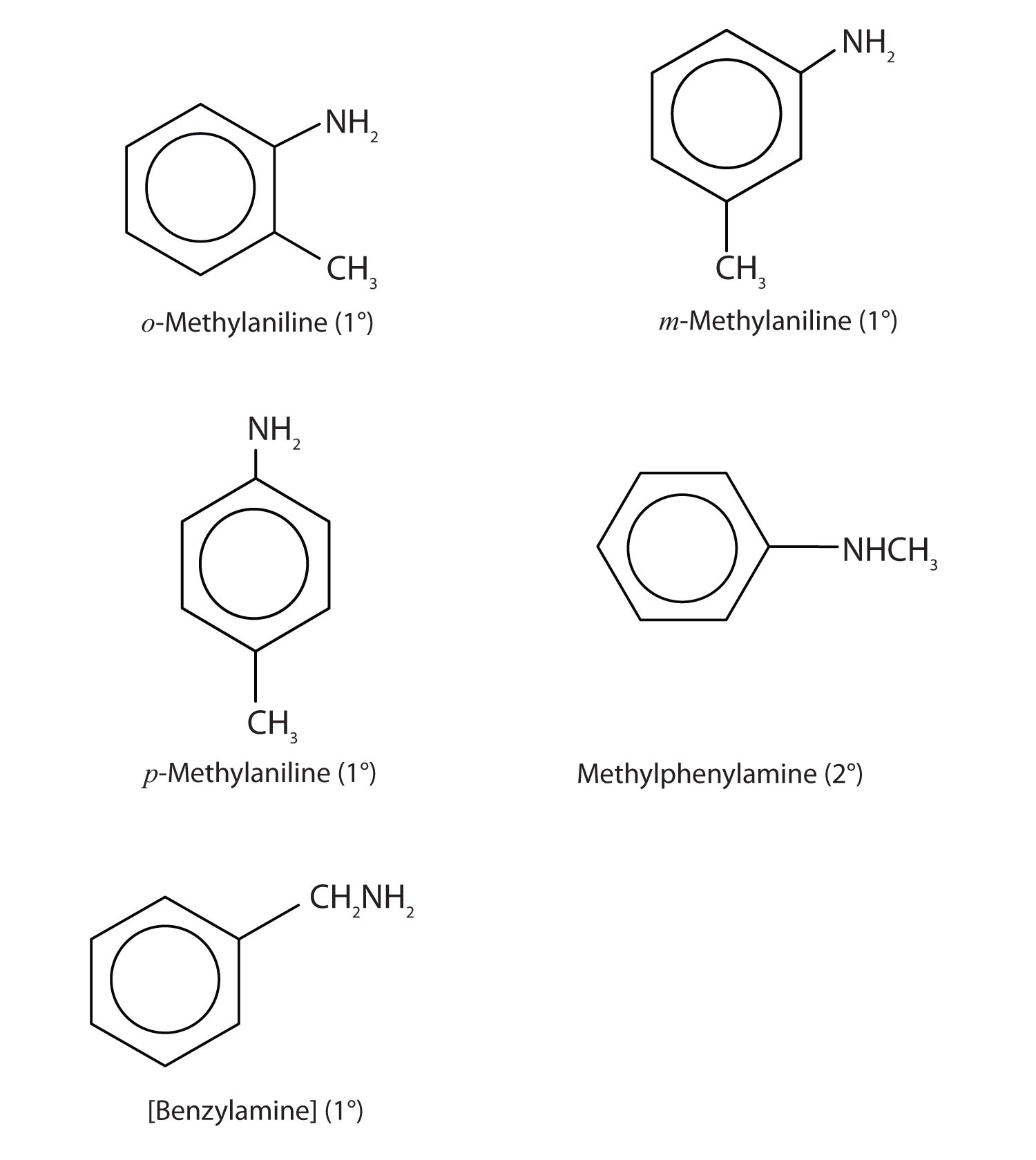
-
-
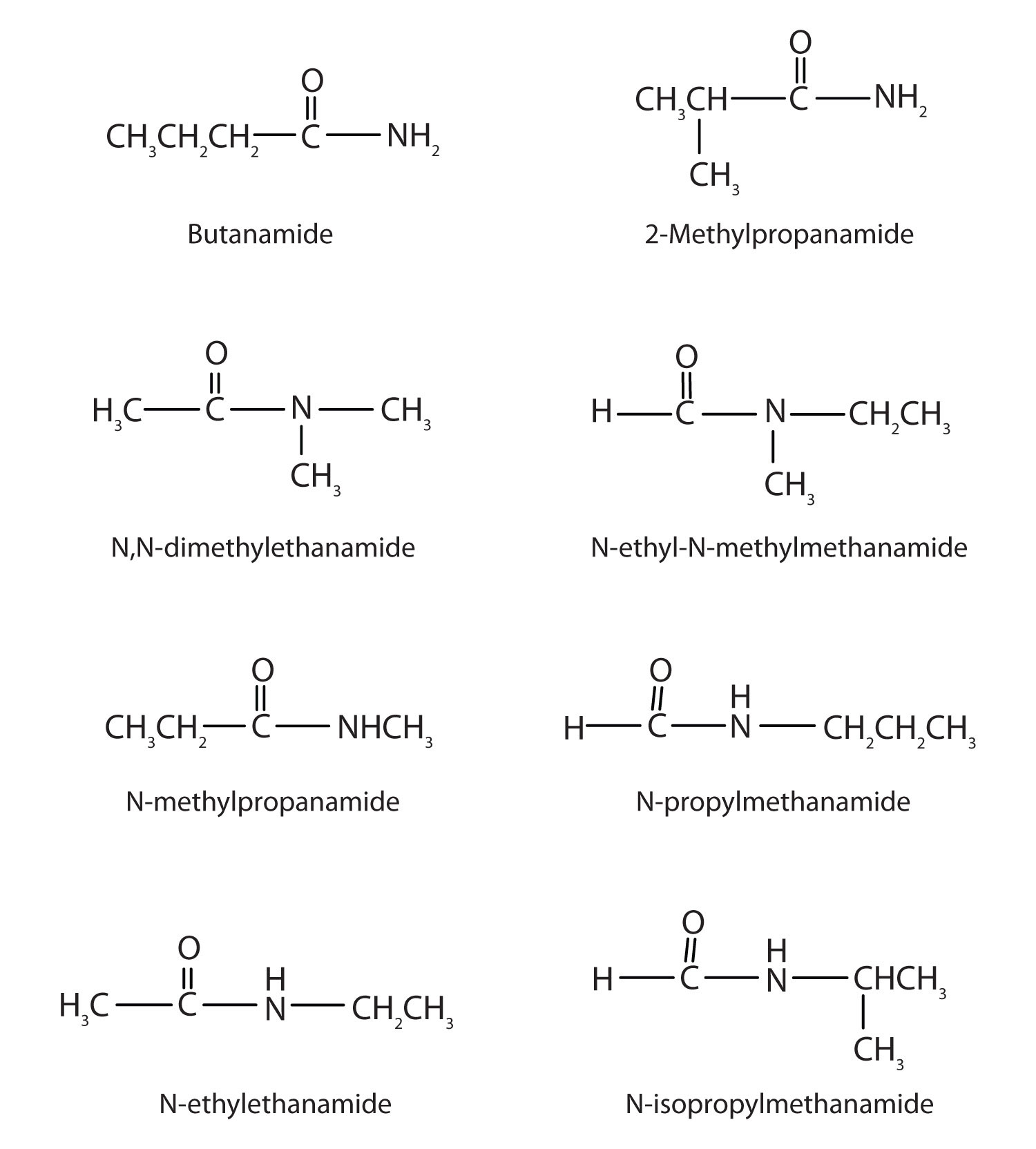
-
-
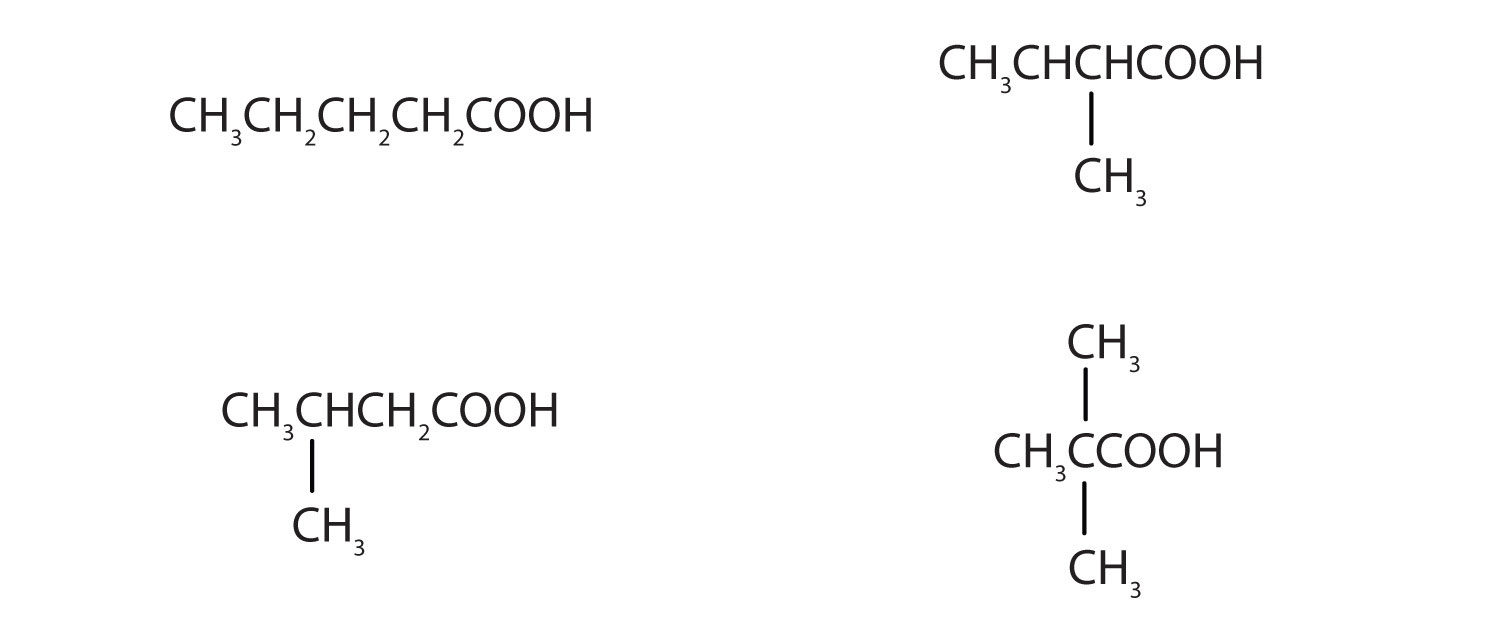
-
-
20.0 mL




