This is “The Formation of Carboxylic Acids”, section 15.3 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
15.3 The Formation of Carboxylic Acids
Learning Objective
- Describe the preparation of carboxylic acids.
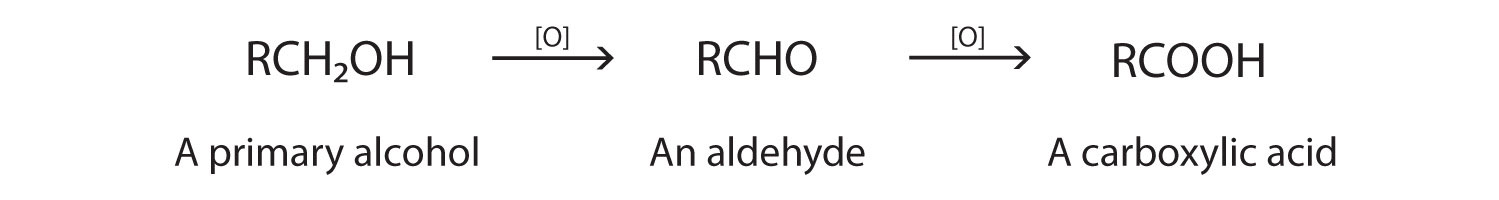
As we noted in Chapter 14 "Organic Compounds of Oxygen", the oxidation of aldehydes or primary alcohols forms carboxylic acids:
In the presence of an oxidizing agent, ethanol is oxidized to acetaldehyde, which is then oxidized to acetic acid.
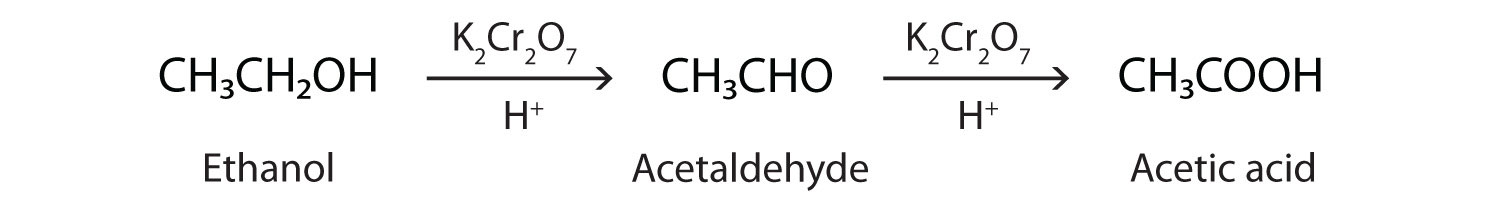
This process also occurs in the liver, where enzymes catalyze the oxidation of ethanol to acetic acid.
Acetic acid can be further oxidized to carbon dioxide and water.
Concept Review Exercises
-
Caproic acid (hexanoic acid) can be prepared in an oxidation reaction from
- what alcohol?
- what aldehyde?
-
Give the structures of the aldehyde and the carboxylic acid formed by the oxidation of isobutyl alcohol [(CH3)2CHCH2OH].
Answers
-
- CH3CH2CH2CH2CH2CH2OH
- CH3CH2CH2CH2CH2CHO
-
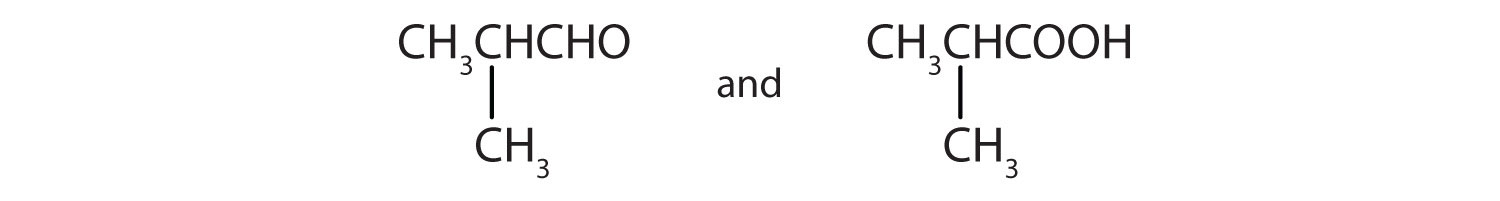
Key Takeaway
- Whether in the laboratory or in the body, the oxidation of aldehydes or primary alcohols forms carboxylic acids.
Exercises
-
Caprylic acid (octanoic acid) can be prepared in an oxidation reaction from
- what alcohol?
- what aldehyde?
-
Give the structures of the aldehyde and the carboxylic acid formed by the oxidation of 1,4-butanediol (HOCH2CH2CH2CH2OH).
Answer
-
- CH3CH2CH2CH2CH2CH2CH2CH2OH
- CH3CH2CH2CH2CH2CH2CH2CHO
-




