This is “Organic Compounds with Functional Groups”, section 14.1 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
14.1 Organic Compounds with Functional Groups
Learning Objective
- Describe functional groups and explain why they are useful in the study of organic chemistry.
In Chapter 12 "Organic Chemistry: Alkanes and Halogenated Hydrocarbons" and Chapter 13 "Unsaturated and Aromatic Hydrocarbons", we considered several kinds of hydrocarbons. Now we examine some of the many organic compounds that contain functional groups. We first introduced the idea of the functional groupA structural arrangement of atoms and/or bonds that imparts a wide range of important properties to organic compounds., a specific structural arrangement of atoms or bonds that imparts a characteristic chemical reactivity to the molecule, in Chapter 4 "Covalent Bonding and Simple Molecular Compounds", Section 4.6 "Introduction to Organic Chemistry". If you understand the behavior of a particular functional group, you will know a great deal about the general properties of that class of compounds. In this chapter and Chapter 15 "Organic Acids and Bases and Some of Their Derivatives", we make a brief yet systematic study of some of organic compound families. Each family is based on a common, simple functional group that contains an oxygen atom or a nitrogen atom. Some common functional groups are listed in Table 14.1 "Selected Organic Functional Groups".
Table 14.1 Selected Organic Functional Groups
| Name of Family | General Formula | Functional Group | Suffix* |
|---|---|---|---|
| alkane | RH | none | -ane |
| alkene | R2C=CR2 |
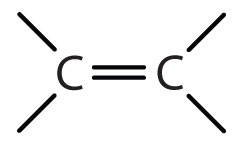
|
-ene |
| alkyne | RC≡CR | –C≡C– | -yne |
| alcohol | ROH | –OH | -ol |
| thiol | RSH | –SH | -thiol |
| ether | ROR | –O– | ether |
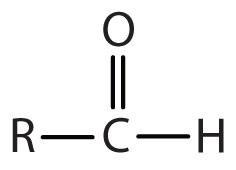
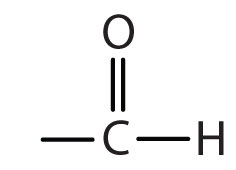
| aldehyde |
|
|
-al |
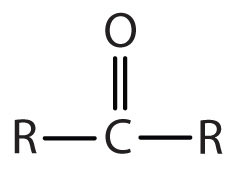
| ketone |
|
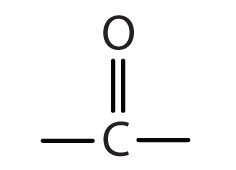
|
-one |
| carboxylic acid |
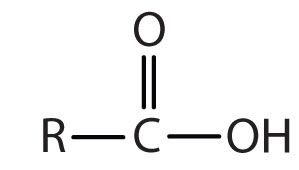
|
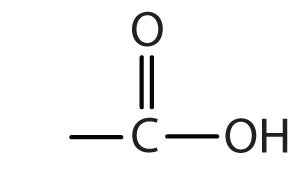
|
-oic acid |
| *Ethers do not have a suffix in their common name; all ethers end with the word ether. | |||
Concept Review Exercises
-
What is the functional group of an alkene? An alkyne?
-
Does CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH3 have a functional group? Explain.
Answers
-
carbon-to-carbon double bond; carbon-to-carbon triple bond
-
No; it has nothing but carbon and hydrogen atoms and all single bonds.
Key Takeaway
- The functional group, a structural arrangement of atoms and/or bonds, is largely responsible for the properties of organic compound families.
Exercises
-
What is the functional group of 1-butanol (CH3CH2CH2CH2OH)?
-
What is the functional group of butyl bromide, CH3CH2CH2CH2Br?
Answer
-
OH
-




