This is “Structures and Names of Alkanes”, section 12.2 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
12.2 Structures and Names of Alkanes
Learning Objective
- Identify and name simple (straight-chain) alkanes given formulas and write formulas for straight-chain alkanes given their names.
We began our study of organic chemistry in Chapter 4 "Covalent Bonding and Simple Molecular Compounds" with the hydrocarbonsThe simplest organic compound, composed of carbon and hydrogen atoms only., the simplest organic compounds, which are composed of carbon and hydrogen atoms only. As we noted, there are several different kinds of hydrocarbons. They are distinguished by the types of bonding between carbon atoms and the properties that result from that bonding. Hydrocarbons with only carbon-to-carbon single bonds (C–C) and existing as a continuous chain of carbon atoms also bonded to hydrogen atoms are called alkanes (or saturated hydrocarbons)A hydrocarbon with only carbon-to-carbon single bonds and existing as a continuous chain of carbon atoms also bonded to hydrogen atoms. Saturated, in this case, means that each carbon atom is bonded to four other atoms (hydrogen or carbon)—the most possible; there are no double or triple bonds in the molecules.
Note
The word saturated has the same meaning for hydrocarbons as it does for the dietary fats and oils: the molecule has no carbon-to-carbon double bonds (C=C). (For more information about fats and oils, see Chapter 17 "Lipids", Section 17.1 "Fatty Acids" and Section 17.2 "Fats and Oils".)
We introduced the three simplest alkanes—methane (CH4), ethane (C2H6), and propane (C3H8)—in Chapter 4 "Covalent Bonding and Simple Molecular Compounds", Section 4.6 "Introduction to Organic Chemistry". They are shown again in Figure 12.1 "The Three Simplest Alkanes". The flat representations shown do not accurately portray bond angles or molecular geometry. Methane has a tetrahedral shape that chemists often portray with wedges indicating bonds coming out toward you and dashed lines indicating bonds that go back away from you. (For more information about the shape of molecules, see Chapter 4 "Covalent Bonding and Simple Molecular Compounds", Section 4.5 "Characteristics of Molecules".) An ordinary solid line indicates a bond in the plane of the page.
Figure 12.1 The Three Simplest Alkanes
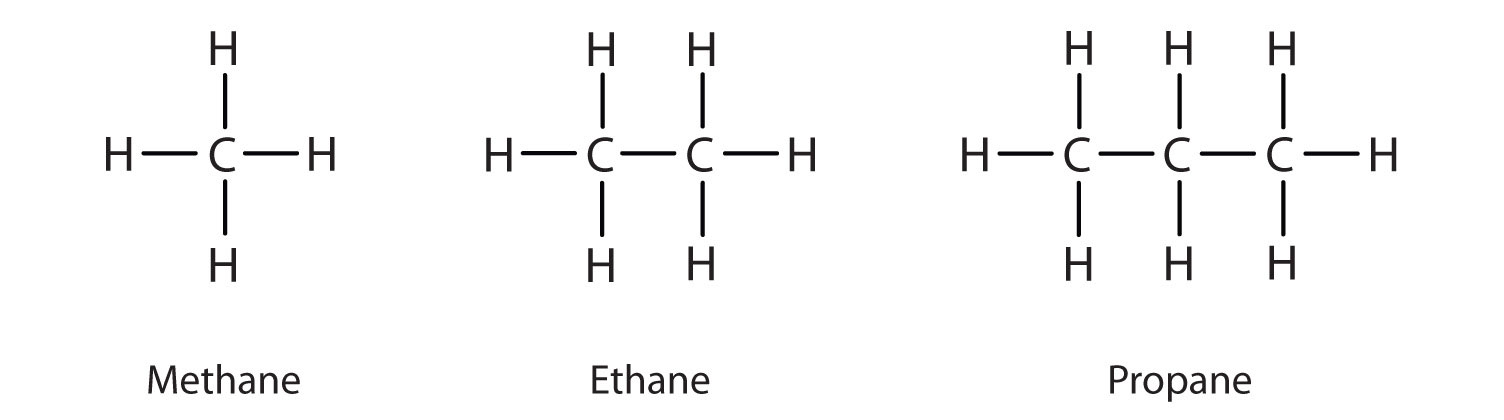
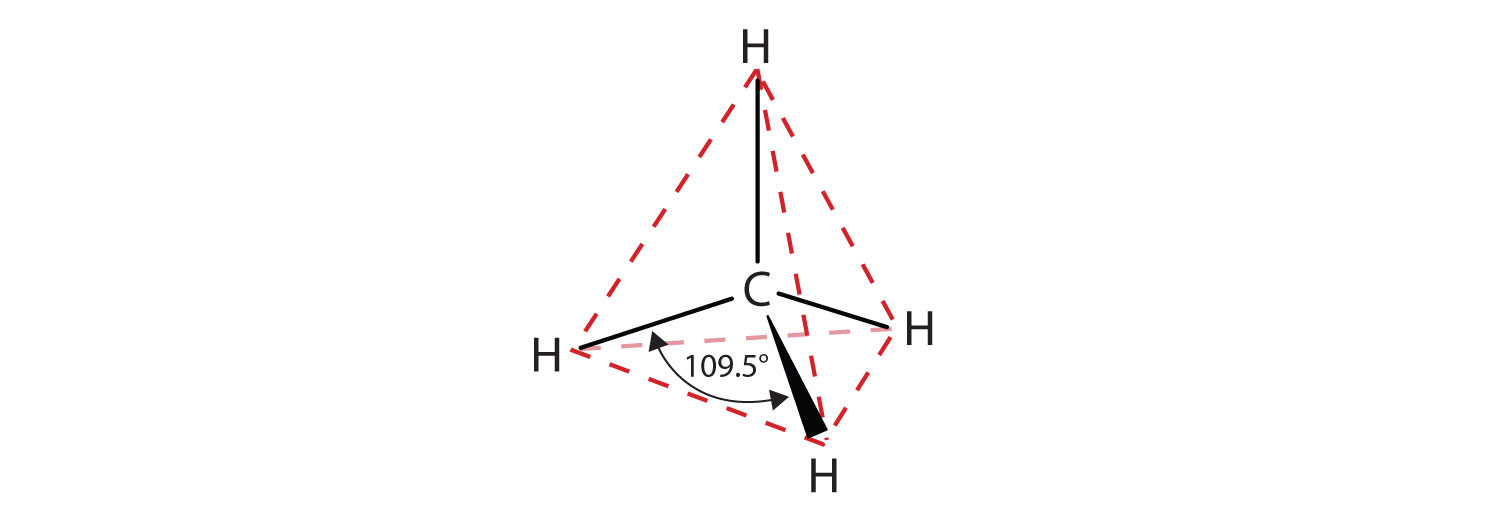
Recall from Chapter 4 "Covalent Bonding and Simple Molecular Compounds", Section 4.5 "Characteristics of Molecules" that the VSEPR theory correctly predicts a tetrahedral shape for the methane molecule (Figure 12.2 "The Tetrahedral Methane Molecule").
Figure 12.2 The Tetrahedral Methane Molecule
Methane (CH4), ethane (C2H6), and propane (C3H8) are the beginning of a series of compounds in which any two members in a sequence differ by one carbon atom and two hydrogen atoms—namely, a CH2 unit. The first 10 members of this series are given in Table 12.2 "The First 10 Straight-Chain Alkanes".
Table 12.2 The First 10 Straight-Chain Alkanes
| Name | Molecular Formula (CnH2n + 2) | Condensed Structural Formula | Number of Possible Isomers |
|---|---|---|---|
| methane | CH4 | CH4 | — |
| ethane | C2H6 | CH3CH3 | — |
| propane | C3H8 | CH3CH2CH3 | — |
| butane | C4H10 | CH3CH2CH2CH3 | 2 |
| pentane | C5H12 | CH3CH2CH2CH2CH3 | 3 |
| hexane | C6H14 | CH3CH2CH2CH2CH2CH3 | 5 |
| heptane | C7H16 | CH3CH2CH2CH2CH2CH2CH3 | 9 |
| octane | C8H18 | CH3CH2CH2CH2CH2CH2CH2CH3 | 18 |
| nonane | C9H20 | CH3CH2CH2CH2CH2CH2CH2CH2CH3 | 35 |
| decane | C10H22 | CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 | 75 |
Consider the series in Figure 12.3 "Members of a Homologous Series". The sequence starts with C3H8, and a CH2 unit is added in each step moving up the series. Any family of compounds in which adjacent members differ from each other by a definite factor (here a CH2 group) is called a homologous seriesAny family of compounds in which adjacent members differ from each other by a definite factor.. The members of such a series, called homologs, have properties that vary in a regular and predictable manner. The principle of homology gives organization to organic chemistry in much the same way that the periodic table gives organization to inorganic chemistry. Instead of a bewildering array of individual carbon compounds, we can study a few members of a homologous series and from them deduce some of the properties of other compounds in the series.
Figure 12.3 Members of a Homologous Series
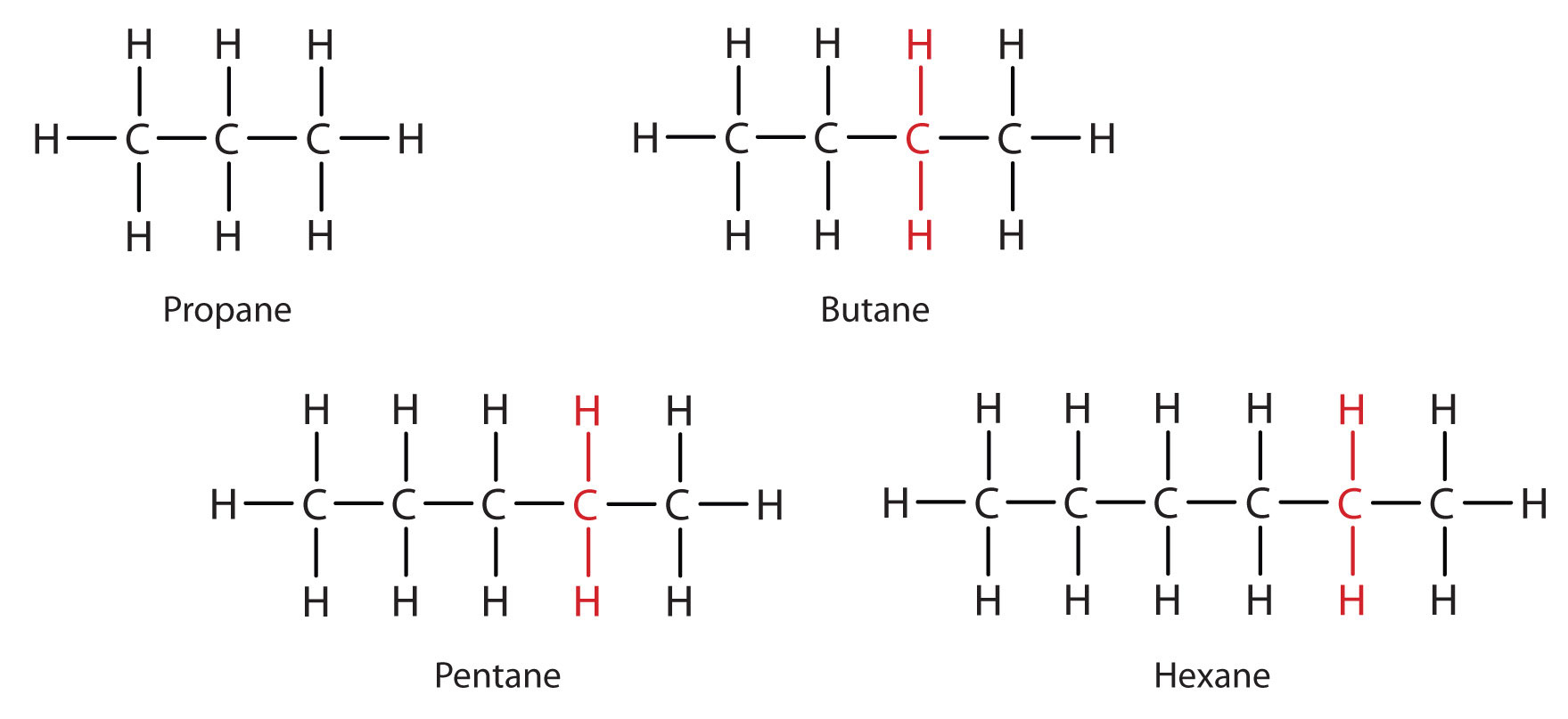
Each succeeding formula incorporates one carbon atom and two hydrogen atoms more than the previous formula.
The principle of homology allows us to write a general formula for alkanes: CnH2n + 2. Using this formula, we can write a molecular formula for any alkane with a given number of carbon atoms. For example, an alkane with eight carbon atoms has the molecular formula C8H(2 × 8) + 2 = C8H18.
Concept Review Exercises
-
In the homologous series of alkanes, what is the molecular formula for the member just above C8H18?
-
Use the general formula for alkanes to write the molecular formula of the alkane with 12 carbon atoms.
Answers
-
C9H20
-
C12H26
Key Takeaway
- Simple alkanes exist as a homologous series, in which adjacent members differ by a CH2 unit.
Exercises
-
What compounds contain fewer carbon atoms than C3H8 and are its homologs?
-
What compounds contain five to eight carbon atoms and are homologs of C4H10?
Answer
-
CH4 and C2H6
-




