This is “The Dissolution Process”, section 9.3 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
9.3 The Dissolution Process
Learning Objective
- Describe the dissolution process at the molecular level.
What occurs at the molecular level to cause a solute to dissolve in a solvent? The answer depends in part on the solute, but there are some similarities common to all solutes.
Recall the rule that like dissolves like. As we saw in Section 9.1 "Solutions", this means that substances must have similar intermolecular forces to form solutions. When a soluble solute is introduced into a solvent, the particles of solute can interact with the particles of solvent. In the case of a solid or liquid solute, the interactions between the solute particles and the solvent particles are so strong that the individual solute particles separate from each other and, surrounded by solvent molecules, enter the solution. (Gaseous solutes already have their constituent particles separated, but the concept of being surrounded by solvent particles still applies.) This process is called solvationThe process by which solute particles are surrounded by solvent particles. and is illustrated in Figure 9.4 "Solvation". When the solvent is water, the word hydrationSolvation by water molecules., rather than solvation, is used.
Figure 9.4 Solvation
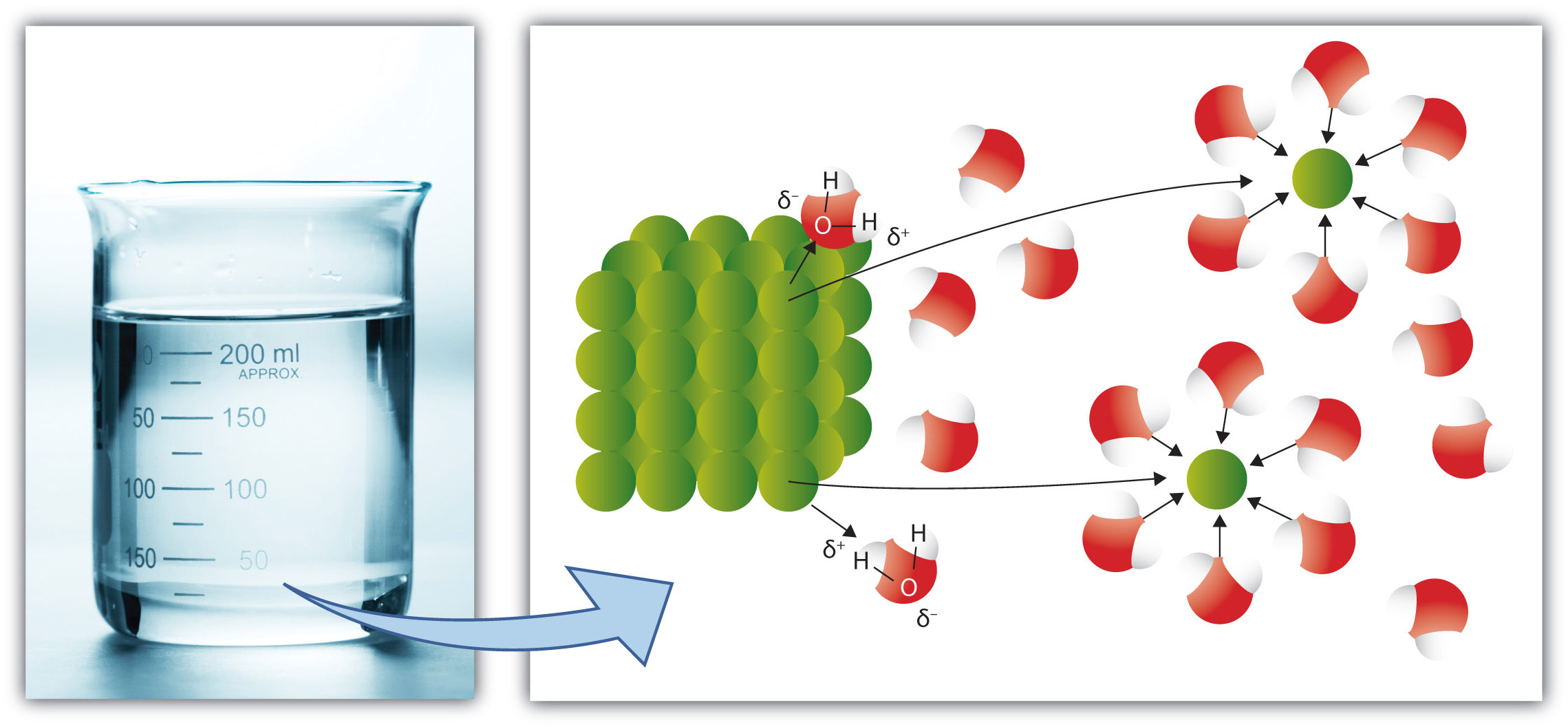
When a solute dissolves, the individual particles of solute become surrounded by solvent particles. Eventually the particle detaches from the remaining solute, surrounded by solvent molecules in solution.
Source: Photo © Thinkstock
In the case of molecular solutes like glucose, the solute particles are individual molecules. However, if the solute is ionic, the individual ions separate from each other and become surrounded by solvent particles. That is, the cations and anions of an ionic solute separate when the solute dissolves. This process is referred to as dissociationThe process of cations and anions of an ionic solute separating when the solute dissolves.. Compare the dissociation of a simple ionic solute as shown in Figure 9.5 "Ionic Dissociation" to the process illustrated in Figure 9.4 "Solvation".
Figure 9.5 Ionic Dissociation
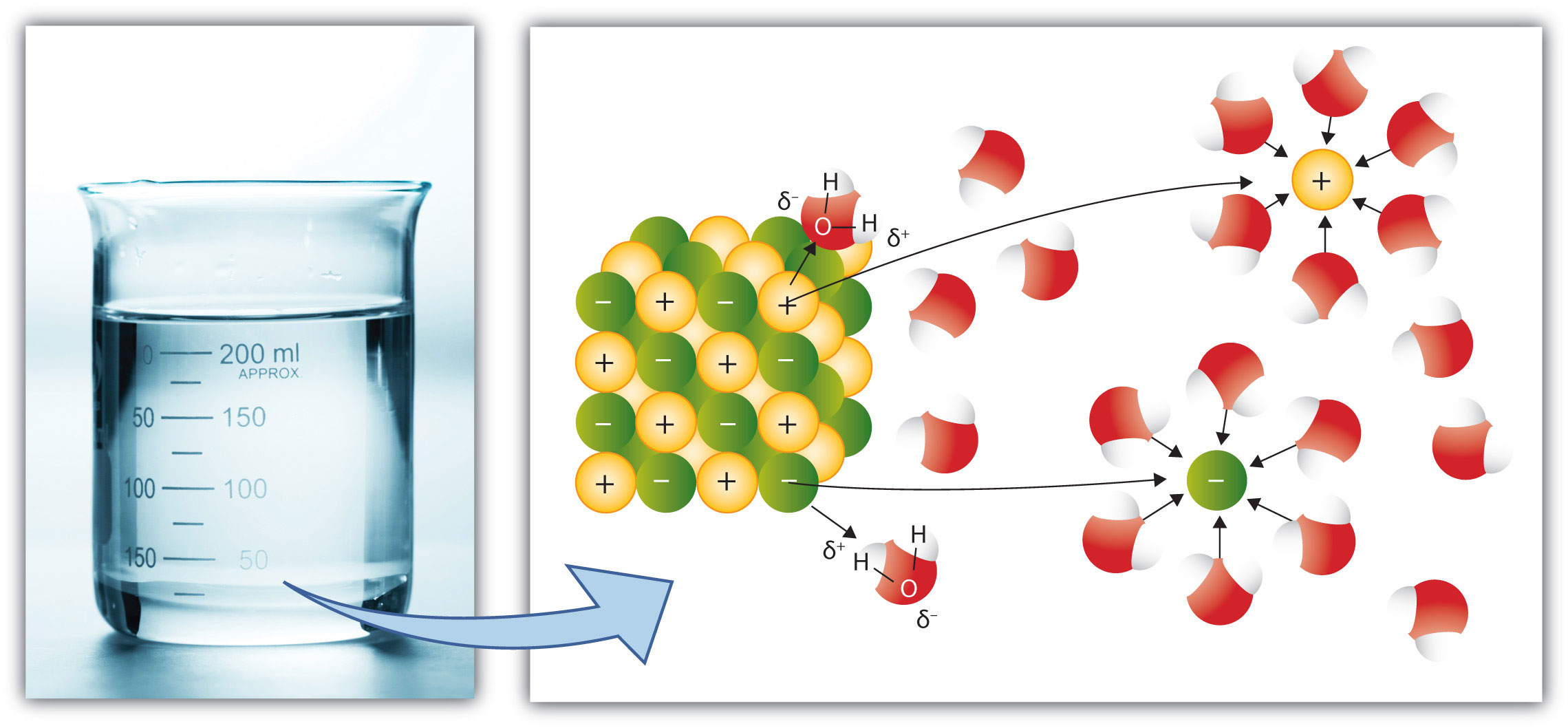
When an ionic solute dissolves, the individual ions separate from each other as they go into solution.
Source: Photo © Thinkstock
The dissociation of soluble ionic compounds gives solutions of these compounds an interesting property: they conduct electricity. Because of this property, soluble ionic compounds are referred to as electrolytesAn ionic compound that dissolves in water.. Many ionic compounds dissociate completely and are therefore called strong electrolytesAn ionic compound that ionizes completely when it dissolves.. Sodium chloride is an example of a strong electrolyte. Some compounds dissolve but dissociate only partially, and solutions of such solutes may conduct electricity only weakly. These solutes are called weak electrolytesAn ionic compound that does not ionize completely when it dissolves.. Acetic acid (CH3COOH), the compound in vinegar, is a weak electrolyte. Solutes that dissolve into individual neutral molecules without dissociation do not impart additional electrical conductivity to their solutions and are called nonelectrolytesA compound that does not ionize at all when it dissolves.. Table sugar (C12H22O11) is an example of a nonelectrolyte.
Note
The term electrolyte is used in medicine to mean any of the important ions that are dissolved in aqueous solution in the body. Important physiological electrolytes include Na+, K+, Ca2+, Mg2+, and Cl−.
Example 10
The following substances all dissolve to some extent in water. Classify each as an electrolyte or a nonelectrolyte.
- potassium chloride (KCl)
- fructose (C6H12O6)
- isopropyl alcohol [CH3CH(OH)CH3]
- magnesium hydroxide [Mg(OH)2]
Solution
Each substance can be classified as an ionic solute or a nonionic solute. Ionic solutes are electrolytes, and nonionic solutes are nonelectrolytes.
- Potassium chloride is an ionic compound; therefore, when it dissolves, its ions separate, making it an electrolyte.
- Fructose is a sugar similar to glucose. (In fact, it has the same molecular formula as glucose.) Because it is a molecular compound, we expect it to be a nonelectrolyte.
- Isopropyl alcohol is an organic molecule containing the alcohol functional group. The bonding in the compound is all covalent, so when isopropyl alcohol dissolves, it separates into individual molecules but not ions. Thus, it is a nonelectrolyte.
- Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Thus, magnesium hydroxide is an electrolyte.
Note
More information than that provided in this chapter is needed to determine if some electrolytes are strong or weak. We will consider this in Chapter 10 "Acids and Bases".
Skill-Building Exercise
-
acetone (CH3COCH3)
-
iron(III) nitrate [Fe(NO3)3]
-
elemental bromine (Br2)
-
sodium hydroxide (NaOH)
The following substances all dissolve to some extent in water. Classify each as an electrolyte or a nonelectrolyte.
Concept Review Exercise
-
Explain how the solvation process describes the dissolution of a solute in a solvent.
Answer
-
Each particle of the solute is surrounded by particles of the solvent, carrying the solute from its original phase.
Key Takeaway
- When a solute dissolves, its individual particles are surrounded by solvent molecules and are separated from each other.
Exercises
-
Describe what happens when an ionic solute like Na2SO4 dissolves in a polar solvent.
-
Describe what happens when a molecular solute like sucrose (C12H22O11) dissolves in a polar solvent.
-
Classify each substance as an electrolyte or a nonelectrolyte. Each substance dissolves in H2O to some extent.
- NH4NO3
- CO2
- NH2CONH2
- HCl
-
Classify each substance as an electrolyte or a nonelectrolyte. Each substance dissolves in H2O to some extent.
- CH3CH2CH2OH
- Ca(CH3CO2)2
- I2
- KOH
-
Will solutions of each solute conduct electricity when dissolved?
- AgNO3
- CHCl3
- BaCl2
- Li2O
-
Will solutions of each solute conduct electricity when dissolved?
- CH3COCH3
- N(CH3)3
- CH3CO2C2H5
- FeCl2
Answers
-
Each ion of the ionic solute is surrounded by particles of solvent, carrying the ion from its associated crystal.
-
-
- electrolyte
- nonelectrolyte
- nonelectrolyte
- electrolyte
-
-
- yes
- no
- yes
- yes
-




