This is “End-of-Chapter Material”, section 3.6 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
3.6 End-of-Chapter Material
Chapter Summary
To ensure that you understand the material in this chapter, you should review the meanings of the following bold terms and ask yourself how they relate to the topics in the chapter.
Atoms combine into compounds by forming chemical bonds. A survey of stable atoms and molecules leads to the octet rule, which says that stable atoms tend to have eight electrons in their outermost, or valence, shell. One way atoms obtain eight electrons in the valence shell is for some atoms to lose electrons while other atoms gain them. When this happens, the atoms take on an electrical charge. Charged atoms are called ions. Ions having opposite charges attract each other. This attraction is called ionic bonding, and the compounds formed are called ionic compounds.
Positively charged ions are called cations, while negatively charged ions are called anions. The formation of both cations and anions can be illustrated using electron configurations. Because elements in a column of the periodic table have the same valence shell electron configuration, atoms in the same column of the periodic table tend to form ions having the same charge. Electron dot diagrams, or Lewis diagrams, can also be used to illustrate the formation of cations and anions.
Ionic compounds are represented in writing by a chemical formula, which gives the lowest ratio of cations and anions present in the compound. In a formula, the symbol of the cation is written first, followed by the symbol of the anion. Formula unit is considered the basic unit of an ionic compound because ionic compounds do not exist as discrete units. Instead, they exist as crystals, three-dimensional arrays of ions, with cations surrounded by anions and anions surrounded by cations. Chemical formulas for ionic compounds are determined by balancing the positive charge from the cation(s) with the negative charge from the anion(s). A subscript to the right of the ion indicates that more than one of that ion is present in the chemical formula.
Some ions are groups of atoms bonded together and having an overall electrical charge. These are called polyatomic ions. Writing formulas with polyatomic ions follows the same rules as with monatomic ions, except that when more than one polyatomic ion is present in a chemical formula, the polyatomic ion is enclosed in parentheses and the subscript is outside the right parenthesis. Ionic compounds typically form between metals and nonmetals or between polyatomic ions.
Names of ionic compounds are derived from the names of the ions, with the name of the cation coming first, followed by the name of the anion. If an element can form cations of different charges, there are two alternate systems for indicating the compound’s name. In the Stock system, a roman numeral in parentheses indicates the charge on the cation. An example is the name for FeCl2, which is iron(II) chloride. In the common system, the suffixes -ous and -ic are used to stand for the lower and higher possible charge of the cation, respectively. These suffixes are attached to a stem representing the element (which frequently comes from the Latin form of the element name). An example is the common name for FeCl2, which is ferrous chloride.
The formula mass of an ionic compound is the sum of the masses of each individual atom in the formula. Care must be taken when calculating formula masses for formulas containing multiple polyatomic ions because the subscript outside the parentheses refers to all the atoms in the polyatomic ion.
Additional Exercises
-
What number shell is the valence electron shell of a sodium atom? What number shell is the valence shell of a sodium ion? Explain the difference.
-
What number shell is the valence electron shell of a bromine atom? What number shell is the valence shell of a bromide ion? Explain the difference between these answers and the answers to Exercise 1.
-
What is the electron configuration of each ion?
- K+
- Mg2+
- F−
- S2−
-
What is the electron configuration of each ion?
- Li+
- Ca2+
- Cl−
- O2−
-
- If a sodium atom were to lose two electrons, what would be the electron configuration of the resulting cation?
- Considering that electron shells are typically separated by large amounts of energy, use your answer to Exercise 5a to suggest why sodium atoms do not form a 2+ cation.
-
- If a chlorine atom were to gain two electrons, what would be the electron configuration of the resulting anion?
- Considering that electron shells are typically separated by large amounts of energy, use your answer to Exercise 6a to suggest why chlorine atoms do not form a 2− anion.
-
Use Lewis diagrams and arrows to show the electron transfer that occurs during the formation of an ionic compound among Mg atoms and F atoms. (Hint: how many atoms of each will you need?)
-
Use Lewis diagrams and arrows to show the electron transfer that occurs during the formation of an ionic compound among K atoms and O atoms. (Hint: how many atoms of each will you need?)
-
Mercury forms two possible cations—Hg2+ and Hg22+, the second of which is actually a two-atom cation with a 2+ charge.
- Using common names, give the probable names of these ions.
- What are the chemical formulas of the ionic compounds these ions make with the oxide ion, O2−?
-
The uranyl ion (UO22+) is a common water-soluble form of uranium. What is the chemical formula of the ionic compound uranyl nitrate? What is the chemical formula of the ionic compound uranyl phosphate?
-
The formal chemical name of the mineral strengite is iron(III) phosphate dihydrate. What is the chemical formula of strengite? What is the formula mass of strengite?
-
What is the formula mass of MgSO4·7H2O?
-
What is the formula mass of CaSO4·½H2O?
-
What mass does 20 formula units of NaCl have?
-
What mass does 75 formula units of K2SO4 have?
-
If an atomic mass unit equals 1.66 × 10−24 g, what is the mass in grams of one formula unit of NaCl?
-
If an atomic mass unit equals 1.66 × 10−24 g, what is the mass in grams of 5.00 × 1022 formula units of NaOH?
-
If an atomic mass unit equals 1.66 × 10−24 g, what is the mass in grams of 3.96 × 1023 formula units of (NH4)2SO4?
-
Both tin and lead acquire 2+ or 4+ charges when they become ions. Use the periodic table to explain why this should not surprise you.
-
Which ion would you expect to be larger in size—In3+ or Tl3+? Explain.
-
Which ion would you expect to be smaller in size—I− or Br−? Explain.
-
Which ion with a 2+ charge has the following electron configuration? 1s22s22p6
-
Which ion with a 3− charge has the following electron configuration? 1s22s22p6
Answers
-
For sodium, the valence shell is the third shell; for the sodium ion, the valence shell is the second shell because it has lost all its third shell electrons.
-
-
- 1s22s22p63s23p6
- 1s22s22p6
- 1s22s22p6
- 1s22s22p63s23p6
-
-
- 1s22s22p5
- It probably requires too much energy to form.
-
-
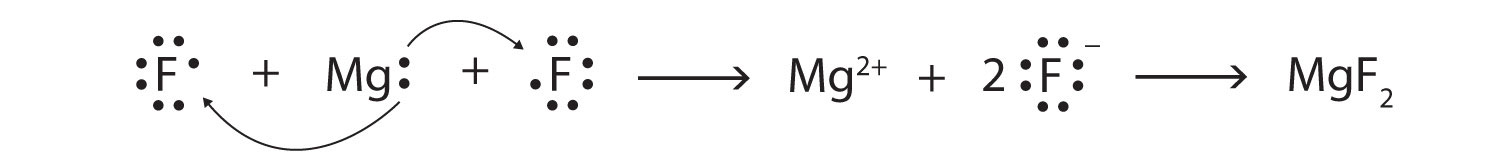
-
-
- mercuric and mercurous, respectively
- HgO and Hg2O, respectively
-
-
FePO4·2H2O; 186.86 u
-
-
145.16 u
-
-
13,070.25 u
-
-
3.32 g
-
-
Both tin and lead have two p electrons and two s electrons in their valence shells.
-
-
Br− because it is higher up on the periodic table
-
-
N3−




